Newsroom
Precise and scarless DNA insertion and replacement represent a core challenge in plant genome editing, with profound significance for crop breeding technologies and food security. The current mainstream precision editing technologies are predominantly based on the prime editing (PE) system. However, the concentrated ownership of their core patents restricts their widespread and flexible application in academic research and industrial practice.
A research team led by Huawei Zhang of Peking University and Jiayang Li of the Institute of Genetics and Developmental Biology of the Chinese Academy of Sciences/Yazhouwan National Laboratory has now introduced a powerful alternative. On November 18, the researchers reported in Molecular Plant a novel genome editing strategy, the "Mortise-Tenon system" (MT), which achieves precise insertion and replacement efficiencies from 16.30% to 59.47% in rice. The strategy offers a new tool for plant genome editing and opening new avenues for crop genetic improvement.
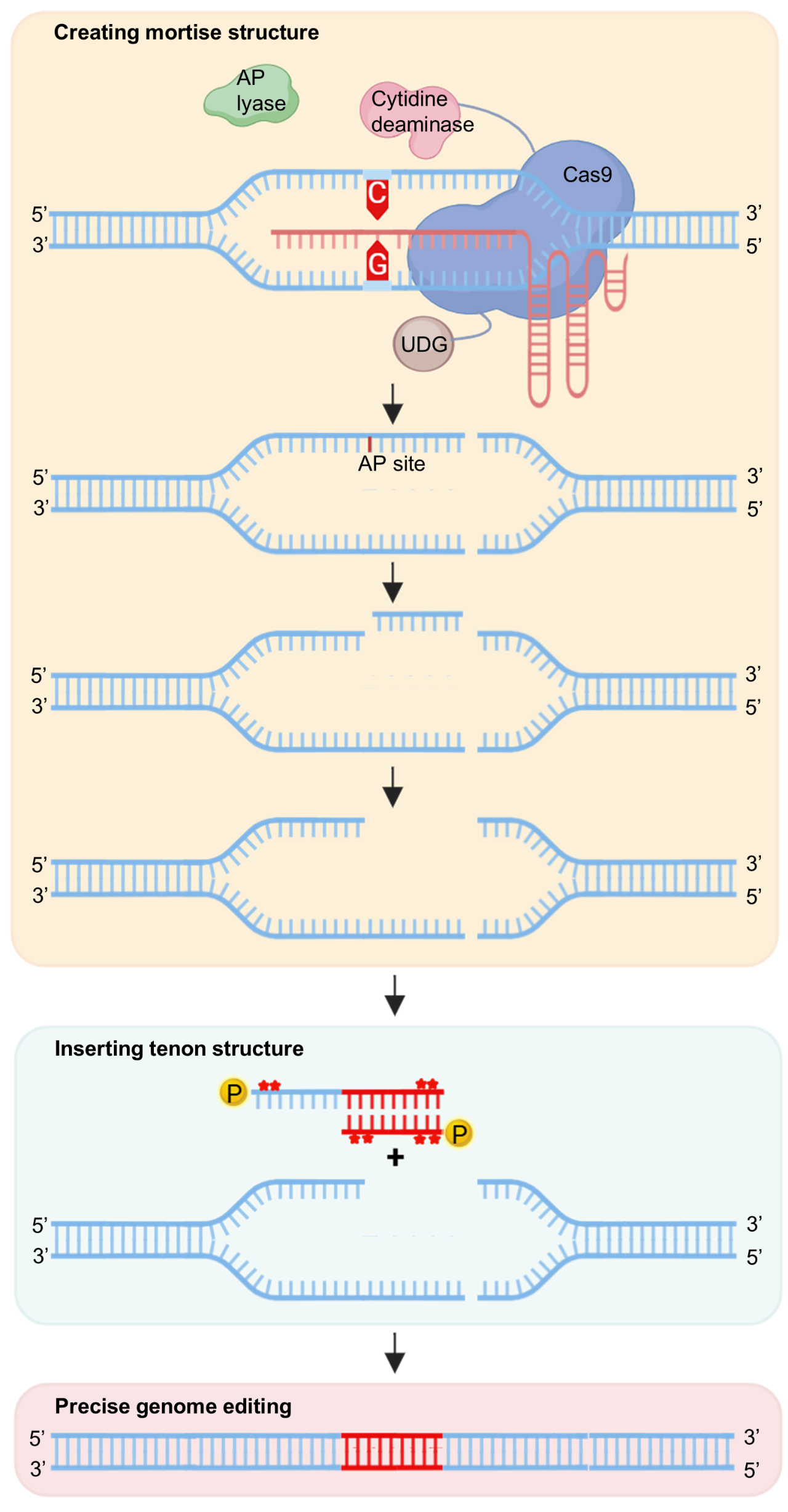
Inspired by the traditional woodworking mortise-tenon structure, the MT system's core lies in the precise complementary pairing of the "mortise" and "tenon." The researchers utilized the APOBEC-Cas9-UDG/AP endonuclease complex from their previously developed AFID system to generate unique double-strand break structures at the target genomic site, such as "mortises" with single-stranded or double-stranded 5' overhangs.
Simultaneously, they designed double-stranded DNA donors with complementary 5' sticky ends as "tenons," enabling precise insertion or replacement of donor fragments through end-capture. The MT system boasts three notable advantages: First, high specificity. The MT2 subsystem leverages APOBEC3B's specificity for TC motifs to generate precisely sized sticky ends, effectively avoiding off-target editing. Second, broad applicability. It can achieve efficient editing for both single and multiple TC motif target sites by designing corresponding sticky-ended donors. Third, comprehensive functionality. It enables precise insertion of 21–85 bp fragments, as well as fragment replacement, with heritable editing events.
Tests at multiple rice gene loci (e.g., GRF1, NRT1.1B, IPA1) demonstrated that the MT system's editing efficiency significantly outperformed traditional Cas9-based methods, reaching up to 59.47%—over 10 times higher than that of conventional Cas9-mediated editor.