
Newsroom
Diacylglycerol O-acyltransferase 1 (DGAT1), the rate-limiting enzyme in triacylglycerol (TAG) biosynthesis, is a crucial target for engineering plants with higher oil yields and improved oil quality. However, the structure of plant DGAT1 and the molecular mechanisms underlying its catalysis and activity regulation have remained unclear.
A research group led by Prof. LIU Zhenfeng from the Institute of Biophysics of the Chinese Academy of Sciences has now resolved high-resolution three-dimensional structures of the wild-type Arabidopsis thaliana DGAT1 (AtDGAT1) and a low-activity mutant (H447A) using single-particle cryo-electron microscopy.
For the first time, the researchers revealed the binding sites for two substrates (DAG and oleoyl-CoA), two products (TAG and CoASH), and multiple free fatty acid (FFA) molecules within DGAT1. The findings were published in The Plant Cell on October 13.
Mutational analysis showed that substitution of Cys246, a residue interacting with the carboxyl head group of FFAs, with alanine, serine, or threonine (C246A/S/T) significantly enhances AtDGAT1 activity.
The cryo-EM structure of the AtDGAT1-H447A mutant displayed pseudo-C2 symmetry, with one monomer binding to the substrates DAG and oleoyl-CoA and the other binding to the products TAG and CoASH.
Structural superimposition of the wild-type and mutant enzymes revealed that an FFA (oleic acid, OLA) molecule helps stabilize the fatty acyl chain and thioester bond of oleoyl-CoA in an orientation favorable for catalysis.
Based on the biochemical and structural data, the researchers proposed a catalytic cycle for acyl transfer mediated by plant DGAT1.
In this model, acyl-CoA is enriched by the N-terminal domain and enters the active site from the cytosolic side, while DAG accesses the site through a lateral membrane opening.
With the help of FFAs, the two substrates are stabilized within the catalytic center. His447 likely deprotonates the hydroxyl group of DAG via a water molecule, enabling a nucleophilic attack on the thioester bond of acyl-CoA to produce TAG and CoASH.
Following a conformational change in the N-terminal domain of the adjacent monomer, CoASH may then be released to the cytosol, whereas TAG exits laterally into the membrane. Finally, DGAT1 returns to its substrate-free initial state.
Through the integration of biochemical and cryo-EM structural analyses, this study sheds light on the catalytic mechanism and FFA-mediated regulation of plant DGAT1 activity. This provides key structural insights for the rational genetic improvement of oilseed crops with higher oil yields and enhanced fatty acid composition.
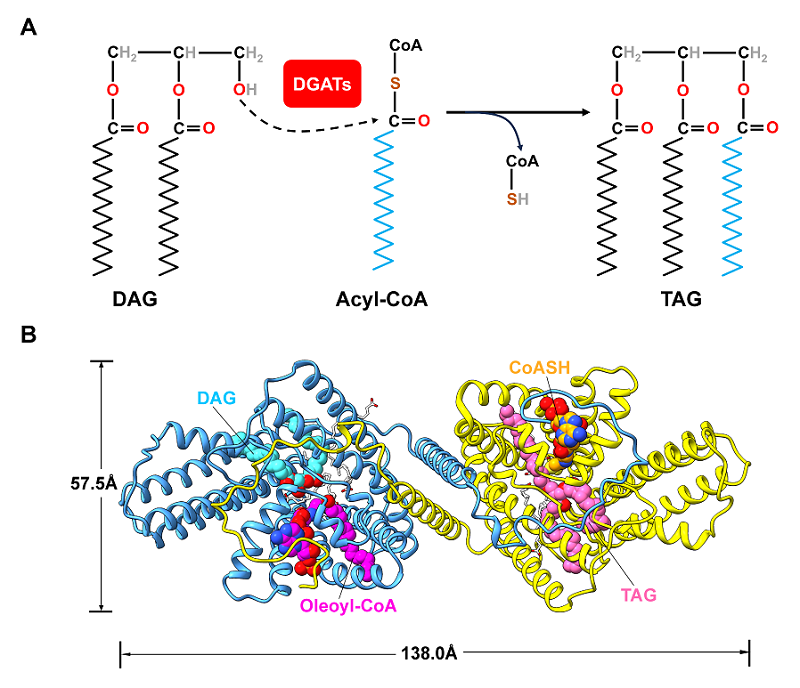
Cryo-EM structures of the AtDGAT1-H447A mutant in complex with OLA, substrates, and products. (Image by LIU Zhenfeng's group)
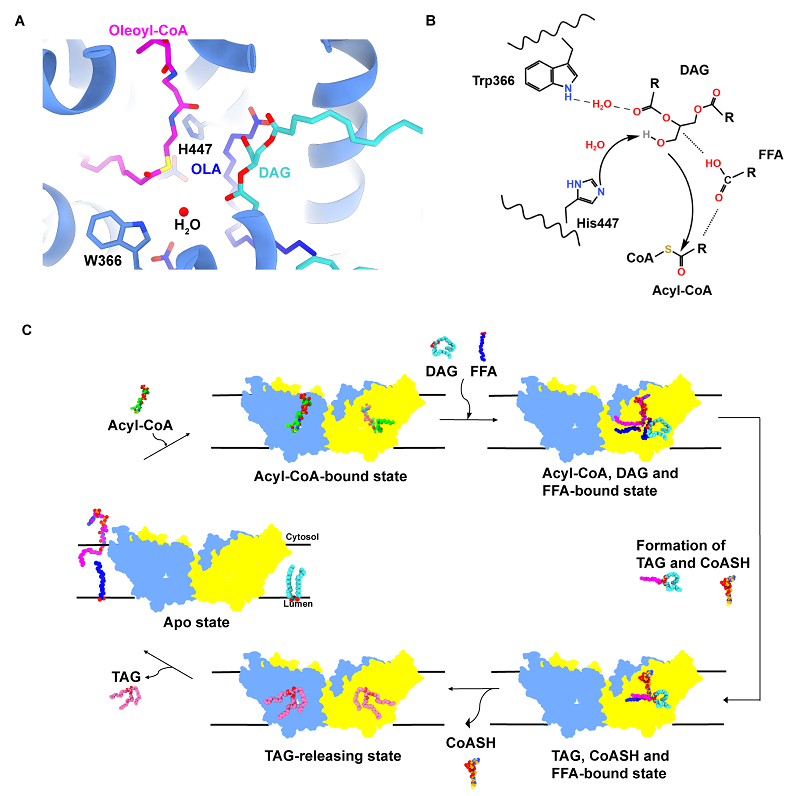
A putative model accounting for the catalytic cycle of DGAT1. (Image by LIU Zhenfeng's group)
