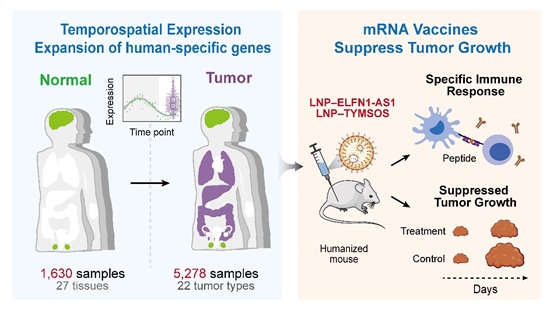
A study led by Dr. LI Chuanyun from the Institute of Genetics and Developmental Biology of the Chinese Academy of Sciences (CAS) has revealed how recently evolved human-specific genes—critical for brain development and cognitive abilities—can be hijacked by cancer to drive tumor growth.
Published in
Cell Genomics on July 17, this study bridges evolutionary biology and cancer medicine, uncovering new therapeutic opportunities.
Unlike most genes that evolve through duplication and modification of existing ones, de novo genes emerge from non-coding DNA, encoding entirely new biological functions. The researchers identified 37 such genes unique to humans and our closest ape relatives based on 120 mammalian genomes, 1,900 human transcriptomes, and 100 million protein spectra. These genes are typically active only in the brain and testes during early development. There they helped shape uniquely human features such as our enlarged brain and improved cognitive capacity over the course of evolution, according to the team's previous studies.
But cancer hijacks these same genes. By analyzing 5,278 tumor samples spanning 22 cancer types, the researchers discovered that nearly half of these genes become aberrantly activated in cancerous tissues—often through the formation of extrachromosomal circular DNA (ecDNA). Functional assays using CRISPR-Cas9 and siRNA confirmed that 57% of these genes directly promote tumor cell proliferation, and their overexpression correlates with worse patient outcomes.
"This is evolution's gamble," said Dr. LI, senior author of the study. "The same genetic innovations that make us smart appear to make us vulnerable, but we are turning this evolutionary ulnerability into clinical opportunity."
The researchers focused on two human-specific genes, ELFN1-AS1 and TYMSOS. "These genes are like dormant bombs," said Dr. LI. "They're completely absent in other species, inactive in healthy adult tissues, but get reactivated only in tumors—making them ideal targets."
In collaboration with Dr. CHENG Qiang of Peking University, the researchers developed mRNA vaccines that train the immune system to recognize these tumor-specific proteins. In humanized mouse models, the vaccines induced robust anti-tumor immune responses, especially when combined with existing immunotherapies. Patient immune cells also showed antigen‑specific immune responses, suggesting a promising new approach.
"These evolutionarily young genes were invisible to cancer research for two key reasons," said co-senior author Dr. AN Ni. "First, they're absent in standard model organisms, and second, genomic databases misannotated them as non-coding or pseudogenes. Our work finally recognizes them as both drivers of human cognition and promising new therapeutic targets—turning millions of years of evolution into potential clinical solutions within years."
This study was supported by CAS and the National Natural Science Foundation of China.
Oncogenic roles of young human de novo genes (Image by IGDB)