
Newsroom
On April 2, researchers from the Institute of Biophysics of the Chinese Academy of Sciences, together with researchers from the Beijing Institute of Technology, published a new study in Nature Communications, unveiling the conformational changes, substrate specificity, and precise molecular mechanism underlying the transport of cyclic dinucleotides (CDNs), folates, and antifolates by SLC19A1.
SLC19A1 is best known for its role in carrying essential nutrients such as folate, as well as antifolate chemotherapy drugs like methotrexate. Recent studies have also revealed that SLC19A1 transports CDNs, small signaling molecules that help activate broad downstream immune responses. However, until now, the details of how SLC19A1 recognizes and transports these diverse molecules remained a mystery.
To address the inherent preference of purified SLC19A1 samples for the inward-open conformation, the researchers developed an innovative antibody-assisted screening strategy for conformationally locked mutants. Using a previously identified inward-open specific antibody, they efficiently screened for the G307F mutant via flow cytometry, successfully stabilizing the protein in the outward-open conformation.
This breakthrough enabled the team to resolve high-resolution structures of SLC19A1 in the outward-open conformation, both unbound and in complex with various substrates: reduced folate (5-MTHF), antifolates (methotrexate and PT523), the effluxed coupling anion (TPP), and a synthetic CDN immunotherapeutic agent (2'3'-CDAS).
Comparative structural analyses, supported by mutational validation, revealed key amino acid residues governing the conformational transitions. In the outward-open state, both folates and antifolates bind as monomers within the central, canonical substrate pocket of SLC19A1. However, differing from the dimeric binding mode observed in the inward-open conformation, CDNs bind as monomers in the canonical outward-open pocket.
Despite certain chemical similarities between folates and CDNs - and their shared binding pocket - their interaction patterns with SLC19A1 are markedly distinct. Systematic mutational analysis revealed that mutations of CDN-specific interacting residues significantly impaired CDN transport without affecting folate uptake, providing a theoretical foundation for the future design of substrate-selective inhibitors.
Furthermore, the study showed that the N-terminal domain of SLC19A1 forms a unique pocket that precisely accommodates the special γ-carboxylate modification present in the novel antifolate PT523. This interaction interface lays a molecular foundation for the development and optimization of next-generation antifolate drugs.
This study not only deepens our understanding of the mechanisms governing multi-substrate transporters but also offers valuable molecular insights for the development of SLC19A1-targeted immunomodulatory therapies and precision antifolate treatments.
Additionally, the dimeric binding mode of CDN in the inward-open conformation presents a promising avenue for the design of specific SLC19A1 inhibitors.
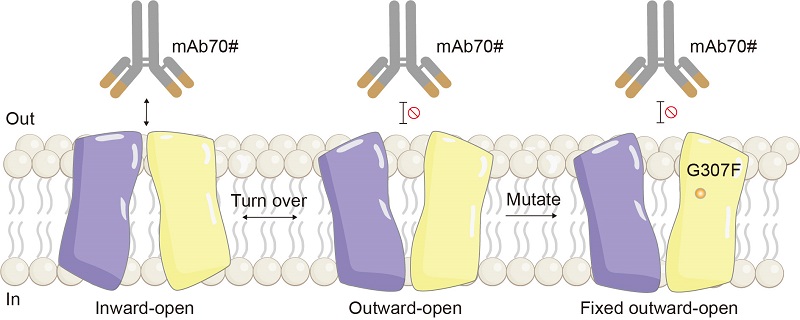
Figure 1. Antibody-assisted screening of outward-open mutants (Image by GAO Pu's group)
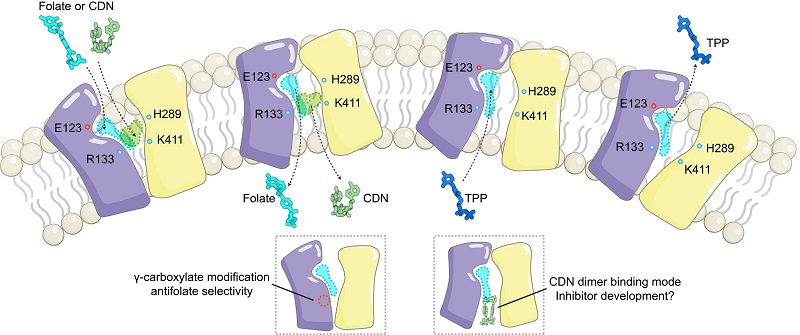
Figure 2. Schematic diagram of SLC19A1 substrate transport (Image by GAO Pu's group)
