
Colorectal cancer (CRC) has emerged as a frequently diagnosed disease. Advanced CRCs are highly heterogenous and often fail the whole treatments, suffering from recurrence and drug resistance.
Fusobacterium nucleatum (F. nucleatum) is among the most prevalent bacterial species in colorectal cancer tissues. The increase of F. nucleatum not only promotes and establishes CRC, but also induces the metastasis and drug resistance. Targeting such cancer-associated microorganisms might provide a new method to abolish drug resistance and ameliorate chemotherapy outcomes.
In a study published in Science Advances, GAO Yuan’s group from the National Center for Nanoscience and Technology (NCNST) of the Chinese Academy of Sciences (CAS) provided an antibiotic-free strategy to inhibit specific bacterial strains for enhanced CRC treatment by designing a nitroreductase (NTR)-instructed supramolecular self-assembly system which can inhibit F. nucleatum and sensitize CRC for chemotherapy.
The researchers synthesized an atypical NTR-responsive motif by introducing additional hydrophilic carboxyl group to the nitrobenzene moiety. The conjugation of the NTR-responsive motif to a suitable peptide sequence yielded an assembly precursor. The precursors underwent NTR catalyzed transformation and formed ordered nanofibers both in vitro and in living systems. These nanofibers rather than the individual molecules exhibited antibacterial activity against F. nucleatum. The local introduction of NTR-instructed assemblies significantly alleviated bacteria-induced oxaliplatin (OXA) resistance effect and efficiently inhibit the tumor growth eventually.
Then, the researchers confirmed that the inhibition of F. nucleatum contributed to the enhanced chemotherapy outcomes. They declared that supramolecular self-assembly could serve as an abiotic strategy to interrupt bacteria-mammalian cell interactions and enhance CRC therapy.
"Such a strategy should be applicable to improve other cancer therapies if the tumors bearing the risk of microbial infections,” said GAO. Taking E. coli associated drug-resistant pancreatic cancer as an example, in such a model, E. coli will metabolize the anticancer drug Gemcitabine into its inactive form, resulting in the failure of chemotherapy against pancreatic cancer.
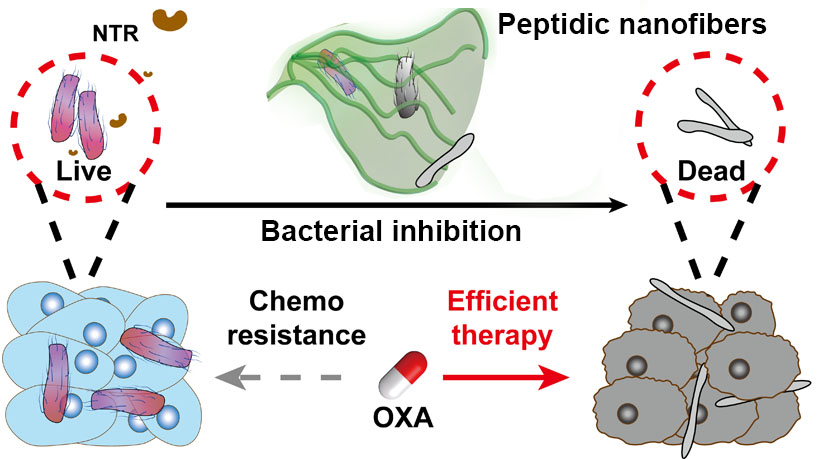
Schematics showing the NTR-instructed peptidic nanofibers with antibacterial activity to inhibit bacterial growth and result in efficient anticancer therapy. (Image by GAO Yuan et al.)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

