
Alkenes are the second most abundant volatile organic compounds (VOCs) in the atmosphere after methane and they primarily originate from anthropogenic and biogenic sources. Alkene ozonolysis produces a carbonyl oxide (also called Criegee intermediates (CIs)) and a carbonyl moiety.
Carbonyl moiety is thought to be an important source of radicals, whose subsequent reactions lead to the formation of hydroperoxides, organic peroxides, and secondary organic aerosols (SOA), thus influencing air quality, climate forcing and human health.
Although SOA are major components of PM2.5 and organic aerosol (OA) particles, the mechanism of SOA formation via Criegee chemistry is poorly understood.
A research group led by Prof. HUANG Yu from the Institute of Earth Environment (IEE) of the Chinese Academy of Sciences used the quantum chemical method to investigate the gas phase reaction mechanism and kinetics of Criegee intermediates (CIs) reactions with hydroxyalkyl hydroperoxides (HHPs) for the first time.
This reaction represented the initial step of oligomer formation and growth during alkene ozonolysis, and therefore needed to be extensively characterized to gain deeper insights into the fundamental chemical composition of these oligomers in the atmosphere.
The calculated results showed that the consecutive reactions of CIs with HHPs were both thermochemically and kinetically favoured, and the oligomers containing CIs as chain units.The addition of -OOH group in HHPs to the central carbon atom of CIs was thermochemically preferable as compared with the -OH group addition pathway (Fig.1).
The barrier height strongly depended on both CI substituent number (one or two) and position (syn- or anti-). The introduction of a methyl group at the anti-position reduced the barrier by ~ 1.0 kcal·mol-1, whereas the corresponding syn-substitutions increased the above barrier by 4.2 kcal·mol-1 (Fig. 2).
The kinetics calculations showed that the introduction of a methyl group into the anti-position significantly increased the rate coefficient, and dramatic decrease was observed when the methyl group was introduced into the syn-position.
This study, published in Atmospheric Chemistry and Physics, highlighted the importance of gas phase oligomerization reaction of carbonyl oxides with HHPs leading to the formation of high-molecular-weight oligomers under atmospheric conditions.

Fig.1 PES (ΔG and ΔE (italic)) for the reaction of CH2OO with HO-CH2OO-H (Pa1) computed at the M06-2X/def2-TZVP//M06-2X/6-311+G(2df,2p) level of theory (Image by CHEN Long, et al)
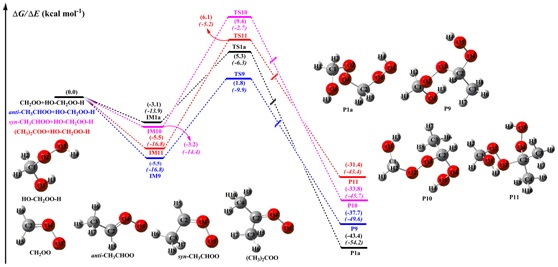
Fig.2 PES (ΔG and ΔE (italic)) of distinct SCI reactions with HO-CH2OO-H (Pa1) calculated at the M06-2X/def2-TZVP//M06-2X/6-311+G(2df,2p) level of theory (Image by CHEN Long, et al)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

