
Drug resistance of tumor cells has been a hot topic in pharmacological and environmental toxicology research. Recently, Prof. YIN Jian and Dr. TIAN Jingjing from Suzhou Institute of Biomedical Engineering and Technology (SIBET) of the Chinese Academy of Sciences, have discovered a new regulatory mechanism of drug resistance proteins in zebrafish embryos.
When an organism encountered the invasion of drugs or chemical contaminants, it will adaptively improve its transformation and efflux ability, thereby degrading or excreting the exogenous substance as soon as possible to achieve self-protection. This effect is also called xenobiotic resistance.
In general, the xenobiotic resistance of an organism relies on multiple protein systems. Among them, the role of adenosine triphosphate-binding cassette (ABC) transporters has received more and more attentions.
ABC transporters is a superfamily containing more than 200 proteins. They are expressed in a series of eukaryotic and prokaryotic organisms, conferring the occurrence of bacterial resistance, blood-brain barrier, placental barrier, drug resistance and toxicity of aquatic animal pollutants.
In particular, the main reason for the failure of chemotherapy treatment is attributed to the high expression of ABC transporters like ABCBs (including Pgp and Bsep, etc.), ABCCs (such as Mrps) and ABCGs (such as Bcrp), which arouses a high drug tolerance by pumping the drug out. Thus, these proteins are also called multidrug resistance proteins.
Scientists used zebrafish embryos as a model to study the modulation mechanism of ABC transporters, with cadmium telluride (CdTe) quantum dots (QDs) as potential environmental toxins.
The results showed that the ABC transporters in zebrafish embryos mediated the efflux and detoxification of QDs. At the same time, it was also found that the nuclear receptor like pregnane X receptor (PXR) and nuclear factor NF-E2-related factor 2 (Nrf2) could be induced when the embryo was harmed, which triggered up-regulation of ABC transporters for detoxicating QDs.
More importantly, the expression of transcription factors changed before ABC transporters, with a level of 10 times higher than that of ABC transporters.
The team's previous results have shown that ABC transporters can recognize and excrete a series of pollutants, including heavy metals, polycyclic aromatic hydrocarbons, and chemotherapeutic drugs such as paclitaxel and doxorubicin.
As an intrinsic regulatory mechanism, nuclear factor has the advantages of being promptly regulated and highly sensitive, which shows potential value as new targets for treating with environmental toxicity and multi-drug resistant tumors.
This paper entitled "Altered Gene expression of ABC transporters, nuclear receptors and oxidative stress signaling in zebrafish embryos exposed to CdTe quantum dots" has been published in Environmental Pollution.
The above work has been supported by the National Science and Technology Major Project of China and National Key R&D Program of China, National Post-Doctor Science Foundation of China and Post-Doctor Foundation of Jiangsu province.
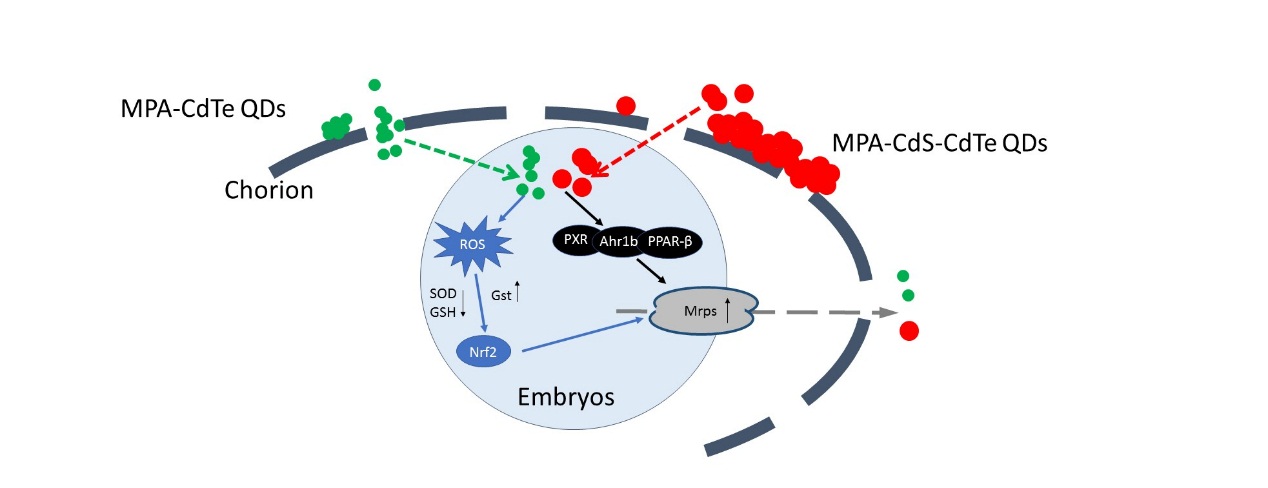
Figure 1: The mechanism of QDs inducing ABC transporters in zebrafish embryos. Both QDs induced the expression of nuclear receptors such as PXR. At the same time, QDs induced the oxidative stress damages in zebrafish embryos and stimulated the expression of Nrf2 factor. The two pathways constituted the internal mechanism for the self-protection of embryos, which functioned by up-regulating the activities of ABC transporters. (Image by YIN Jian)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

