
Glycosylation of therapeutic IgG antibodies plays an important role in immunoregulating effect via interaction with Fc receptors. Glycoengineering of IgG with optimal Fc N-glycans indicates improved efficacy in antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC).
Prof. HUANG Wei's group from Shanghai Institute of Materia Medica of the Chinese Academy of Sciences recently provided a new strategy for glycosite-specific antibody-drug conjugates via IgG glycoengineering.
In this study, an approach of chemoenzymatic IgG glycan remodeling catalyzed by an Endo-glycosidase called Endo-S has been developed for efficient IgG glycoengineering. The finding has been published in Nature Protocols.
Based on previous technology of chemoenzymatic IgG glycan remodeling, Prpf. HUANG's group used a series of unnatural glycan substrates to assemble azido tags to IgG glycosites, thereafter enables the small-molecule drug conjugates in a site-specific manner.
Previous study revealed that antibody-drug conjugates (ADCs) carries a highly potent cytotoxin covalently linked on IgG antibodies and selectively delivers the toxin into tumor cells through antibody-antigen specific recognition.
Conventional ADCs connect the toxin to lysine or cysteine residues of IgG randomly, therefore brings heterogeneity issue in ADC quality control and pharmacokinetic consistence. However, this study shows that the glycosite-specific ADCs features with excellent homogeneity in molecular structure and minimizes the influence of IgG chemical modification on antigen-binding affinity.
"Glycosite-specific conjugation is also valuable in labeling of commercial antibody bio-reagents." Prof. HUANG said. Currently, thousands of antibody bio-reagents were widely used in western blotting, immunoprecipitation, immunofluorescence, immunohistochemistry, ELISA, and other basic biotechnologies. Randomly labeling of antibodies might cause inconsistence in experimental results by possible modification on the antigen-binding site of antibodies. However, using this method, Fc glycosite labeling, could solve this problem easily.
This work was supported by Chinese "1000 Young Talent" Plan, Chinese Academy of Sciences, and National Natural Science Foundation of China.
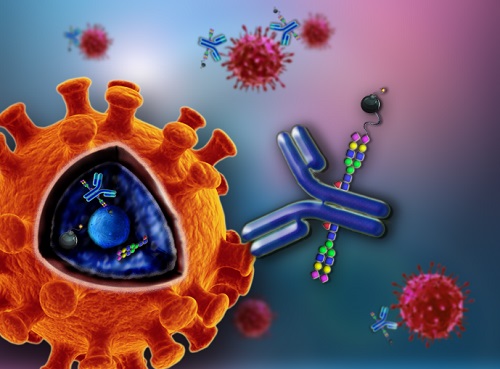
Glycosite-specific antibody-drug conjugates for tumor-targeting therapy (Image by HUANG Wei)

86-10-68597521 (day)
86-10-68597289 (night)

86-10-68511095 (day)
86-10-68512458 (night)

cas_en@cas.cn

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

