NMPA Conditionally Approves Gumarontinib for Treatment of Non-Small Cell Lung Cancer with MET Exon 14 Skipping Mutation
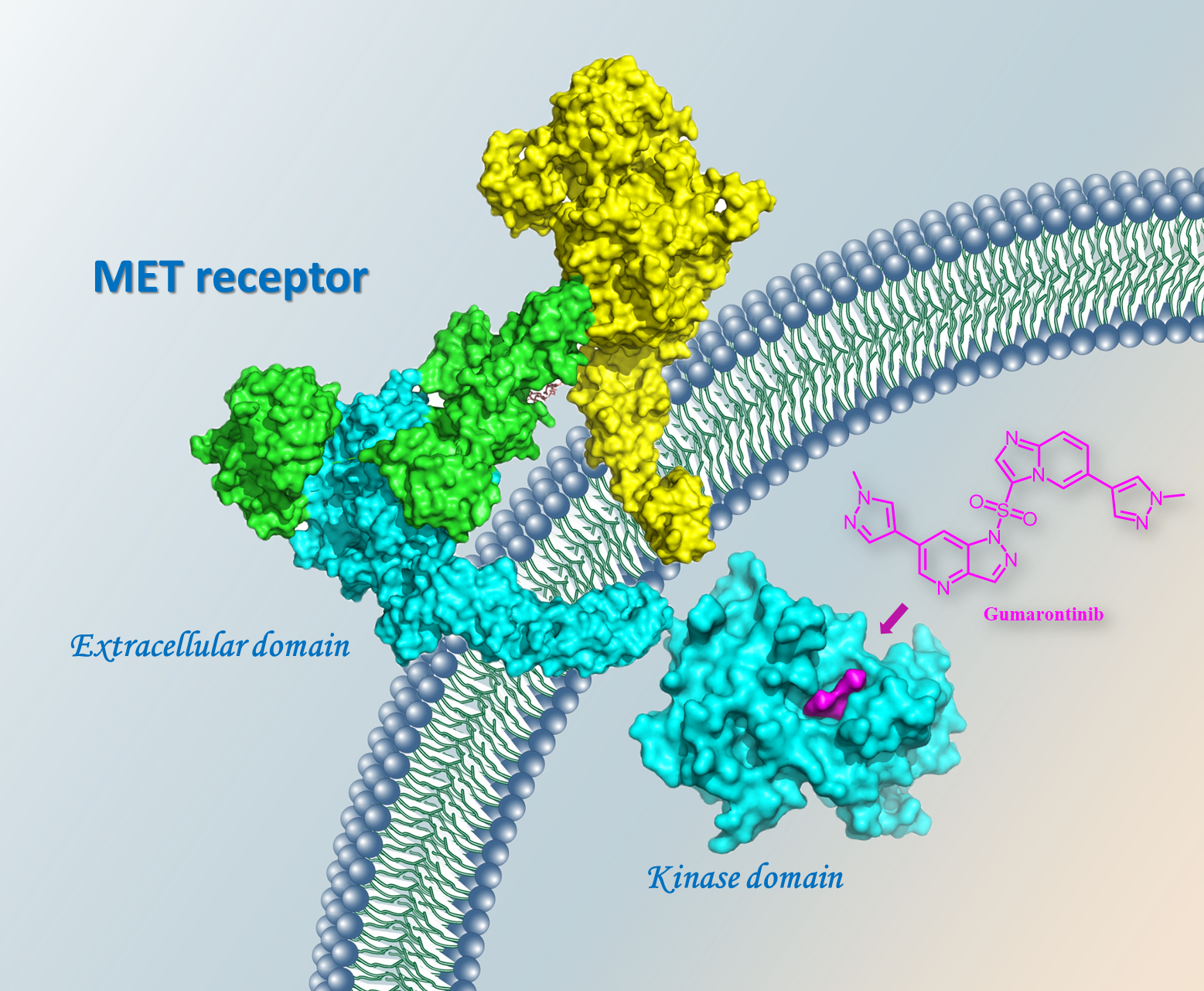
Gumarontinib, an oral highly selective mesenchymal-epithelial transition (MET) inhibitor and a Category 1 innovative new chemical drug, for the treatment of adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) with MET exon 14 skipping mutation got conditionally approval on March 8, 2023, by the National Medical Products Administration (NMPA) of China.
Mar 09, 2023