
Newsroom
A recent study led by Prof. ZHENG Hui from the Guangzhou Institutes of Biomedicine and Health of the Chinese Academy of Sciences has revealed a novel mechanism by which cell cycle regulation influences epigenetic modifications, facilitating the reprogramming of somatic cells into induced pluripotent stem cells (iPSCs). This research, published in Advanced Science, offers new insights into cell fate transitions.
The study developed enhanced transcription factors by fusing the activation domain from the herpesvirus VP16 to the transcription factors OCT4 and SOX2, resulting in the creation of the OvSvK complex (OCT4-VP16/SOX2-VP16/KLF4). Compared to the classical OSK (OCT4/SOX2/KLF4) combination, OvSvK induced germline-competent iPSCs by day four of reprogramming—significantly increasing efficiency.
Single-cell analyses showed that OvSvK restructures the cell cycle by shortening the pre-DNA synthesis phase (G1 phase) while extending the DNA synthesis phase (S phase), creating an embryonic stem cell-like pattern. siRNA screening identified a complementary combination (CCC), which involved co-overexpressing Ccne1 while suppressing Ccnd1 and Cdkn2a. The CCC similarly modified the cell cycle and accelerated reprogramming, further validating the critical role of cell cycle regulation.
Comparative analysis of the OSK and OvSvK systems revealed that genes highly expressed in the OvSvK system—but not directly regulated by OCT4 or SOX2—showed reduced levels of H3K27me3 (trimethylation of histone H3 at lysine 27). Notably, these patterns were also observed in the CCC system, indicating that restructuring the cell cycle affects epigenetic heritability. While previous studies established that epigenetic marks require the S-G2/M transition for restoration in daughter cell G1 phases, their roles in regulating cell fate had not been fully understood. Utilizing the Fucci system alongside image-based profiling, this study demonstrated that the G1 shortening induced by OvSvK inhibits the restoration of H3K27me3, which in turn elevates gene expression.
In conclusion, this study reveals that OvSvK accelerates reprogramming through dual mechanisms: the VP16 fusion enhances direct OS-target expression, while cell cycle restructuring modulates H3K27me3-mediated regulation of non-OS targets. These findings establish cell cycle dynamics as key epigenetic modulators of cell fate transitions, providing fundamental insights into the mechanisms of reprogramming.
This work was supported by the National Key Research and Development Program of China, and the National Natural Science Foundation of China, among other sources.
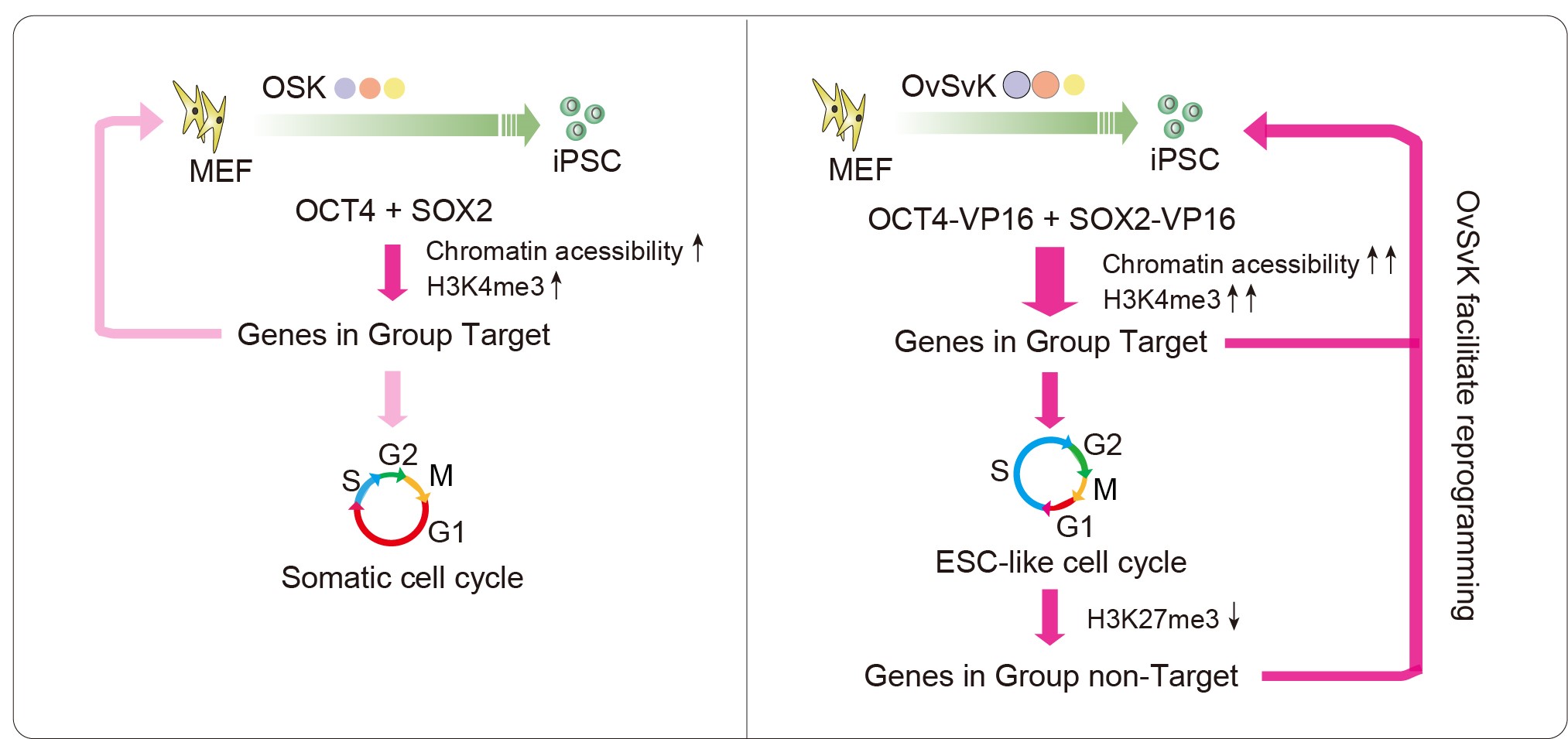
Schematic of the OvSvK reprogramming mechanism (Image by Prof. ZHENG's team)
