
Recently, a proof-of-concept validation clinical trial for a new personalized drug treatment technology (named HDS) initiated by a research group in Hefei Institutes of physical science and a clinical team in the first affiliated hospital of Chongqing Medical University released the interesting positive results.
This retrospective clinical trial data demonstrated that compared to conventional treatment, patients guided by HDS technology showed 8 months longer the median progression-free survival (PFS).
According to the latest data released by the National Cancer Center in China in 2019, the annual incidence of liver cancer in China is 20 to 40 per 100,000 people, accounting for more than 50% of new cases worldwide. So far, treatment for liver cancer is limited, especially for patients with advanced disease progression.
Due to the lack of safe, effective and precision therapies, there are still urgent clinical demand for the new technology. Therefore, it is important to maximize the value of the drugs in the existing clinical practice guidelines.
LIU Qingsong's team at CASHIPS developed a new technology, "high-throughput drug sensitivity (HDS) testing technology", it is based on the breakthrough of their key technique for in-vitro expansion of patient-derived tumor cells.
The technology could provide scientific decision-making guidance for cancer patients, especially those refractory patients.
A clinical team led by Dr. SHI Zhengrong in the Department of Liver Surgery, the First Affiliated Hospital of Chongqing Medical University, adopted the individualized drug treatments guided by HDS technology for patients with the postoperative recurrence compared with patients with conventional therapy.
After observation and data analysis of more than 170 patients with recurrent liver cancer, the results showed that the overall median DFS of the HDS technique guided group was significantly better than the conventional treatment group (17.00±3.80 vs 9.00±1.05 months, P=0.001).
This is the first validation trial of in-vitro drug sensitivity testing using patient-derived tumor cells to guide the clinical treatment for liver cancer. The success of this validation trial is also expected to speed up the promotion of personalized drug treatment technology, to provide cancer patients, especially those in the advanced stage, more drug treatment options.
The trial was sponsored by Precedo Pharmaceutics Inc. Hefei, Anhui, China.
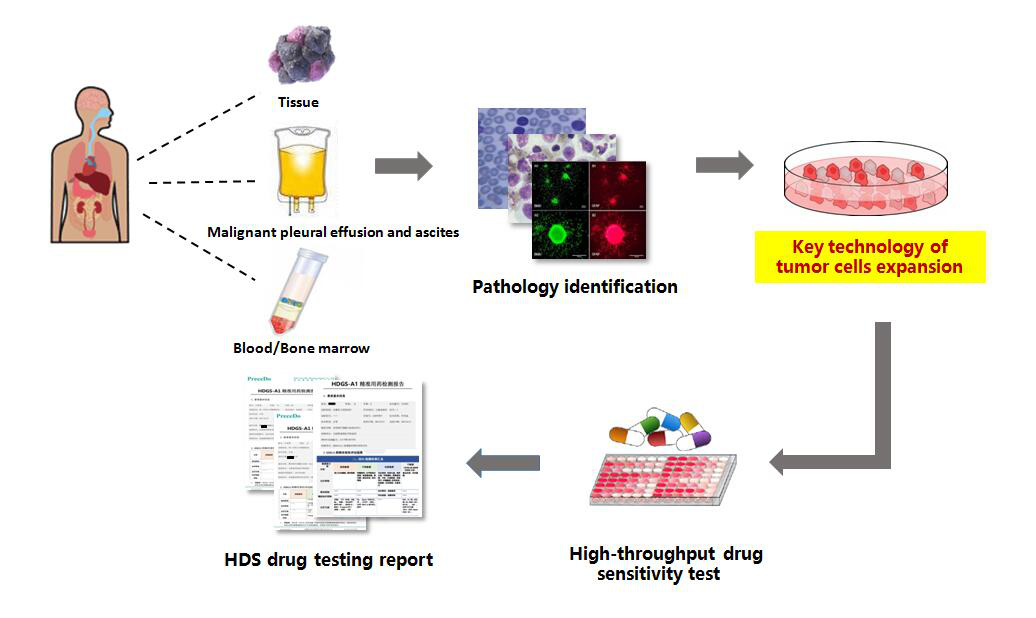
Flow chart of HDS test technology (Image by LIU Feiyang)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

