
Newsroom
Cytotoxic lymphocytes eliminate target cells through apoptosis and pyroptosis, an inflammatory form of programmed cell death. Granzyme A (GZMA)-mediated activation of gasdermin B (GSDMB) is a key mechanism of pyroptotic killing, how GZMA specifically recognizes and cleaves GSDMB has remained unknown.
In a new study published in Immunity on January 26, researchers from the Institute of Biophysics of the Chinese Academy of Sciences and the Beijing Institute for Life Sciences, uncovered the precise mechanism by which cytotoxic lymphocyte-derived GZMA induces target cell pyroptosis.
The researchers first discovered that GZMA binds to the C-terminal autoinhibitory domain of GSDMB with high affinity, forming a stable enzyme-substrate complex. Then, they determined the crystal structure of the GZMA-GSDMB C-terminal domain complex.
This structure clearly shows that GZMA engages two GSDMB C-terminal domains symmetrically in a dimeric configuration. The groove along the dimer interface acts as an exosite, spatially separated from the GZMA catalytic center, and specifically recognizes the binding site on GSDMB formed by the L1 and L2 loops.
Structural superposition of the full-length autoinhibited GSDMB onto the complex reveals that exosite-mediated recognition positions the cleavage site (Lys244) at the linker between the two GSDMB domains in close proximity to the catalytic center of one GZMA protomer, enabling highly specific proteolytic cleavage of GSDMB.
Since exosite formation strictly depends on GZMA dimerization, mutational and functional analyses demonstrated that disrupting dimer formation or impairing the exosite at the dimer interface eliminates GZMA's ability to recognize and cleave GSDMB. Consequently, this prevents GSDMB-dependent pyroptosis.
The study also showed that although mouse GZMA maintains conserved dimeric architecture and catalytic machinery, its exosite contains altered key residues necessary for GSDMB recognition, resulting in loss of specificity and efficient cleavage toward GSDMB. Rationally re-engineering the exosite of mouse GZMA conferred robust activity toward GSDMB, enabling efficient cleavage and activation of the pyroptotic effector.
This work is the first to structurally elucidate a granzyme in complex with its physiological substrate. It identifies a novel exosite and a unique dimeric feature that underlie GZMA-specific recognition of GSDMB. Additionally, it reveals the precise mechanism by which GZMA activates GSDMB to induce target-cell pyroptosis.
Single-nucleotide polymorphisms in the GSDMB locus are closely associated with autoimmune and inflammatory diseases, including asthma and inflammatory bowel disease. Since rodents naturally lack the GSDMB gene, combining exosite-engineered mouse GZMA with GSDMB transgenic models offers valuable tools for probing the physiological and pathological functions of the GZMA-GSDMB axis in vivo.
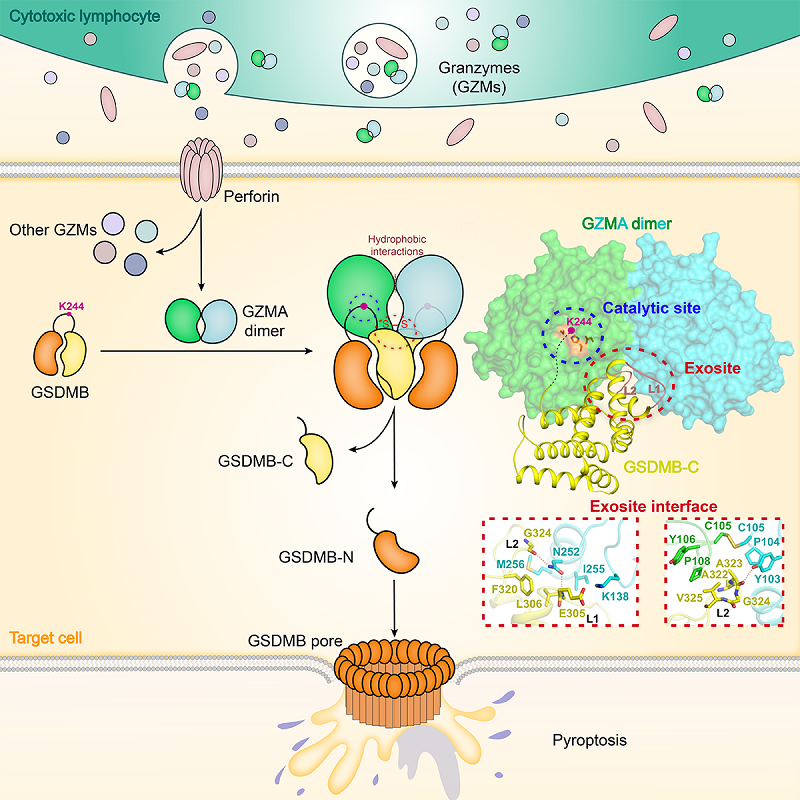
Schematic diagram of exosite-mediated targeting of GSDMB by dimeric granzyme A in lymphocyte pyroptotic killing (Image by DING Jingjin's group)
