
Newsroom
Researchers from the Institute of Biophysics of the Chinese Academy of Sciences have uncovered the molecular mechanisms that govern the function and inhibition of the human glucose-6-phosphate transporter 1 (G6PT1), a central regulator of hepatic glucose production and a key player in both inherited metabolic disorders and diabetes therapy.
Using an integrated cryo-electron microscopy (cryo-EM) single-particle approach, the researchers led by Prof. ZHAO Yan from the Institute of Biophysics of the Chinese Academy of Sciences have determined the high-resolution three-dimensional structure of full-length, wild-type human G6PT1 in multiple functional states.
The study was published in Science Advances on January 31.
According to the researchers, these structures include the inward-facing apo conformation; the inward-facing conformation bound to the substrate, glucose-6-phosphate (G6P); the outward-facing conformation bound to the cosubstrate, inorganic phosphate (Pi); and the inward-facing conformation bound to the natural inhibitor, 2-deoxy-D-glucose (CGA). This series of structures provides a comprehensive, atomic-resolution view of the transport and inhibition mechanisms of G6PT1.
The researchers demonstrated that the negatively charged G6P molecule is accommodated within a highly positively charged central binding pocket of G6PT1, precisely coordinated by multiple key amino acid residues. Mutation of these residues severely compromises or completely abolishes transport activity. Several clinically frequent GSD1b-causing mutations (e.g., R28C/H, W118R, and W138R) cluster in this pocket. This provides a direct structural explanation for how disruption of substrate binding leads to disease.
Further structural analyses reveal that in the outward-facing conformation, the phosphate group of G6P closely overlaps with the Pi binding site and shares key coordinating residues. This indicates a competitive binding relationship between Pi and G6P. Subtle rearrangements of the substrate-binding pocket during conformational transitions favor the release of G6P into the endoplasmic reticulum (ER) lumen. This offers direct structural evidence for the classical "Pi/G6P antiport" model.
The study also demonstrates that CGA binds to G6PT1 in the inward-facing conformation in a manner resembling a "molecular wedge," partially occupying the G6P binding site while strongly stabilizing this conformation. By preventing the transition to the outward-facing state, CGA effectively locks the transport cycle. The nonconservation of CGA's binding residues among other SLC37 family members provides a structural basis for developing highly selective G6PT1 inhibitors.
These findings establish a new framework for the rational design of G6PT1-targeted therapeutics for type 2 diabetes, paving the way for the development of next-generation antidiabetic agents with improved potency, selectivity, and pharmacokinetic properties.
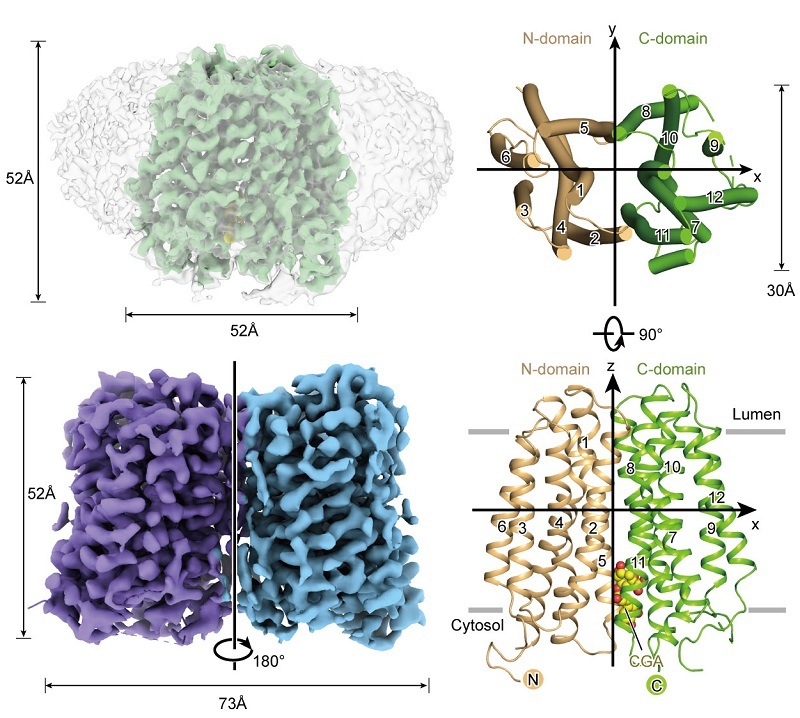
Figure 1. Overall Structure of G6PT1 (Image by ZHAO Yan's group)
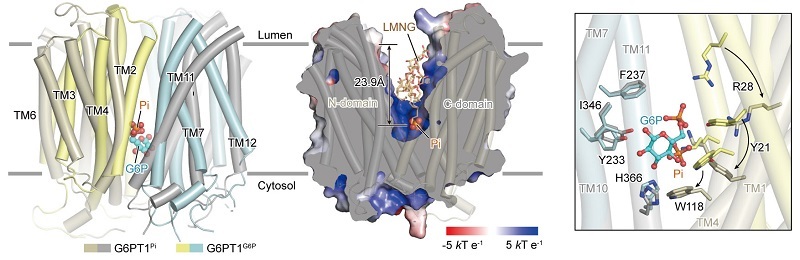
Figure 2. Substrate Recognition and Transport Mechanism of G6PT1 (Image by ZHAO Yan's group)
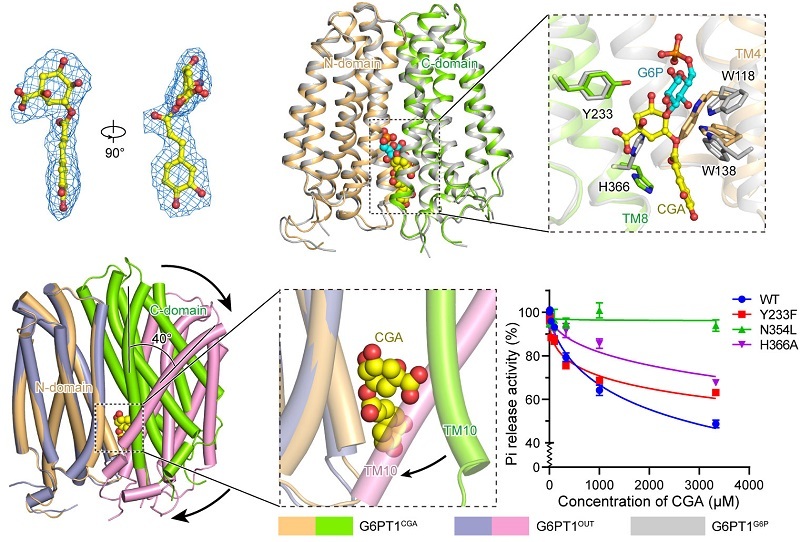
Figure 3. Molecular Mechanism of G6PT1 Inhibition by CGA (Image by ZHAO Yan's group)
