
Newsroom
The constant vibration/rotation and hydrogen-bond (HB) rearrangement of water molecules create various complex yet dynamic HB networks, which makes the characterization of the structure of liquid water become difficult.
Inasmuch as the nature of intermolecular forces between water molecules in water clusters bears resemblance to that in the bulk, spectroscopic studies of water clusters reveal the basic building blocks of the HB network, and provides central benchmarks for developing accurate potential functions and universal models of water.
In a study published in Nature Communications, a research team led by Prof. JIANG Ling and Prof. LI Gang from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences, along with Prof. LI Jun from Tsinghua University, experimentally determined the structure motifs of water undecamer cluster (H2O)11.
Prof. JIANG Ling and Prof. LI Gang proposed a method of infrared (IR) spectroscopy of neutral clusters based on a tunable vacuum ultraviolet free electron laser (VUV-FEL). They applied this method to neutral (H2O)11, and recorded the IR spectrum with diverse bands.
By combining IR spectrum with high-precision quantum chemical computations developed by Prof. LI Jun's group, three lowest-energy configurations denoted as 515, 43'4, and 55'1 structural motifs were determined. These structures corresponded to the "5+1+5", "4+3+4", and "5+5+1" assembling of water cluster pairs, respectively, with the 515 configuration being dominant.
Furthermore, through thermodynamic analysis, the researchers revealed the cluster growth mechanisms from water decamer clusters.
This study offers crucial insights into the evolution of water's HB network, and paves the way for size-dependent studies to explore the stepwise mechanisms of solvation processes such as salt dissolution and acid dissociation.
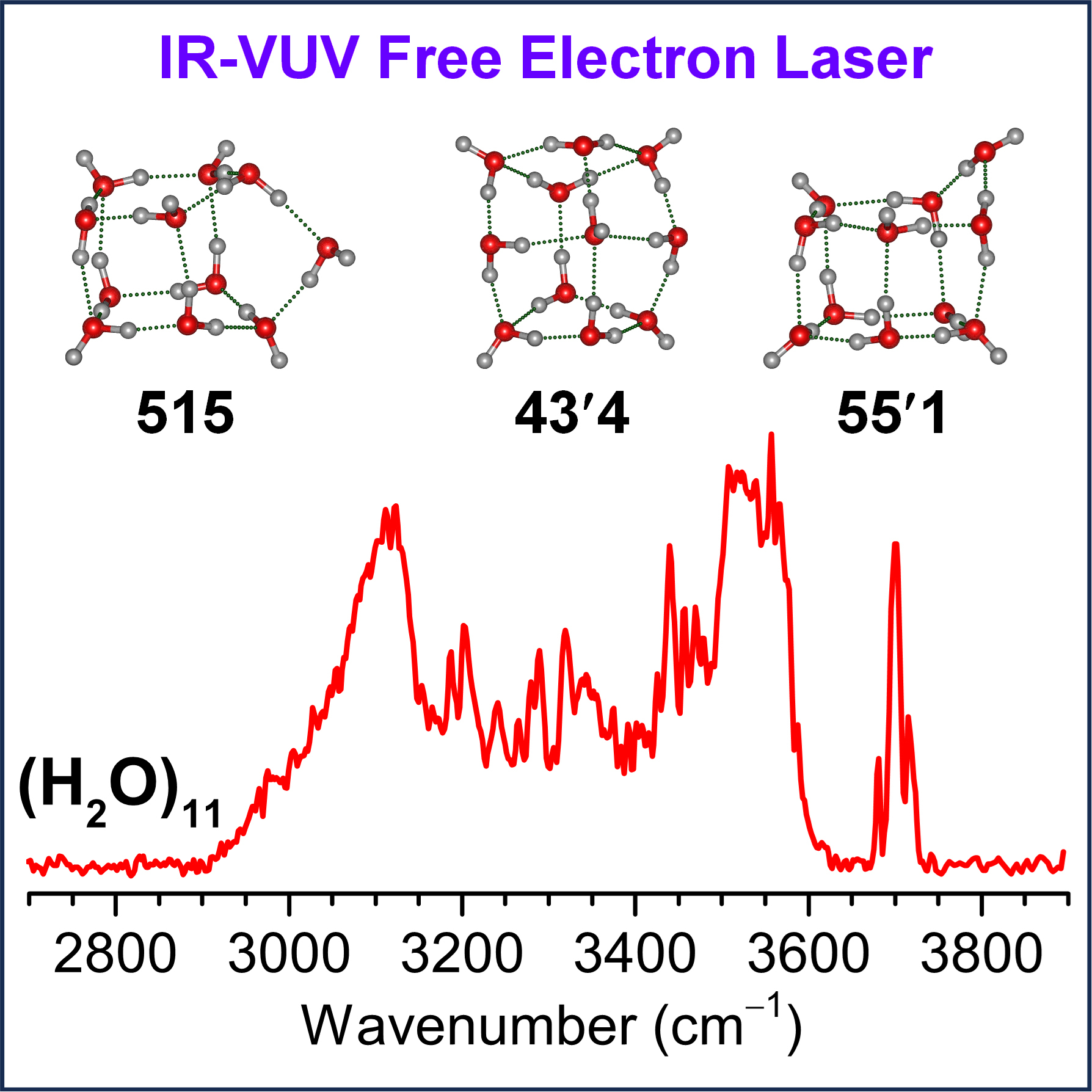
Experimental IR spectrum and the identified structures of water undecamer cluster (H2O)11. (Image by WANG Tiantong)
