
Newsroom
Solanesyl diphosphate synthase (SPS) is a key enzyme in the biosynthetic pathway of plastoquinone, a photosynthetic electron carrier. It has recently been identified as a novel target of herbicides. Fibrillin 5 (FBN5) is believed to play a role in plastoquinone biosynthesis by modulating the catalytic activity of SPS. However, the molecular basis of the interaction between FBN5 and SPS, as well as how FBN5 modulates SPS catalysis, remains unclear.
In a new collaborative study, researchers from the Institute of Biophysics of the Chinese Academy of Sciences, and Central China Normal University systematically elucidated the catalytic mechanism of OsSPS3 in rice (Oryza sativa) and the molecular basis of its interaction with OsFBN5 by combining biochemical analyses, structural biology and computational simulations.
The findings, published in Nature Plants on January 5, provide a solid framework for understanding the regulation of plastoquinone biosynthesis.
The researchers first determined high-resolution crystal structures of OsSPS3 bound to various ligands. The asymmetric dimeric architecture of OsSPS3 suggests that the enzyme may achieve catalysis through conformational switching between the two monomers in an alternating manner.
Using a combination of complementary experimental approaches, the researchers then demonstrated a strong and highly specific direct interaction between OsFBN5 and the chloroplast-localized OsSPS3. They subsequently solved high-resolution cryo-electron microscopy structures of the OsSPS3-FBN5 complex in both the apo state and in complex with the substrate analogue GGSPP.
Based on these structural insights, the researchers proposed a molecular model in which OsFBN5 induces OsSPS3 to transition from an alternating to a synchronous catalytic mechanism.
This mechanism not only explains how OsFBN5 regulates OsSPS3 function but also provides direct experimental evidence for the marked enhancement of overall catalytic efficiency by OsFBN5.
Based on the resolved high-resolution structures, the researchers further performed virtual screening and identified the clinically used anti-osteoporosis drug zoledronate (ZOL) as a compound that can effectively bind to the catalytic pocket of SPS.
In vitro enzymatic assays and crystal structure analyses revealed the binding mode of ZOL as a competitive inhibitor. Greenhouse herbicidal activity assays showed that ZOL exhibits broad-spectrum and high-efficiency herbicidal activity, indicating its potential as a lead compound for the development of new SPS-targeting herbicides.
This study advances our understanding of the catalytic mechanisms of photosynthesis-related enzymes, providing important insights for molecular breeding. It also lays a solid foundation for developing novel SPS-based herbicides.
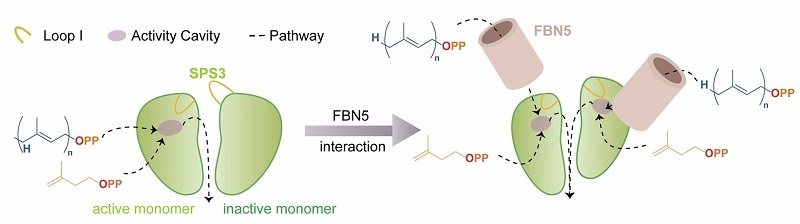
Molecular model illustrating FBN5-induced transition of SPS3 from alternating to synchronous catalysis (Image by ZHU Ping's group)
