
2024
Mogrosides, a class of secondary metabolites that are most present in the fruit extract of monk fruit, constitute a series of natural sweeteners and are known for their high sweetness and low calorie content and thus have high market potential in food additives.
However, their wide utilization is limited by a variety of factors including low yield of Monk fruit, small amount of sweet glycosides in the fruit, and high extraction cost. Biosynthesis is a promising solution to obtain high yield and high purity mogrosides.
Recently, a collaborative study between the Institute of Biophysics of the Chinese Academy of Sciences and the Institute of Medicinal Plants of the Chinese Academy of Medical Sciences reported multiple complex structures and enzymatic characteristics of the glycosyltransferase SgUGT94-289-3.
SgUGT94-289-3 is a key enzyme in the biosynthesis of mogrosides. It constitutes a uridine diphosphate (UDP)-dependent glycosyltransferase (UGT) responsible for the biosynthesis of mogroside V (M5) and siamenoside I (SIA), the two mogrosides with high intensities of sweetness.
The researchers found that SgUGT94-289-3 forms a dual-substrate pocket at its active site. This allows the two structurally distinct reaction ends of mogrosides to be delivered from different pockets to the active site for glycosylation reactions, thereby maintaining catalytic region selectivity while achieving substrate promiscuity.
Through structure-based directed engineering, the researchers further identified critical structural motifs essential for catalytic activity and regioselectivity, leading to the development of SgUGT94-289-3 mutants with significantly increased M5/SIA yields.
This study provides an in-depth revelation of the dual-pocket substrate binding model of SgUGT94-289-3, enriches the understanding of the molecular mechanisms underlying asymmetric substrate promiscuity recognition in glycosyltransferases, and offers valuable structural information for multi-step enzyme engineering reactions catalyzed by single enzymes.
Published in Nature Communications, this work revealed the structural and functional details of SgUGT94-289-3 and thus laid the foundation for designing engineered SgUGT94-289-3 enzymes with higher activity and selectivity.
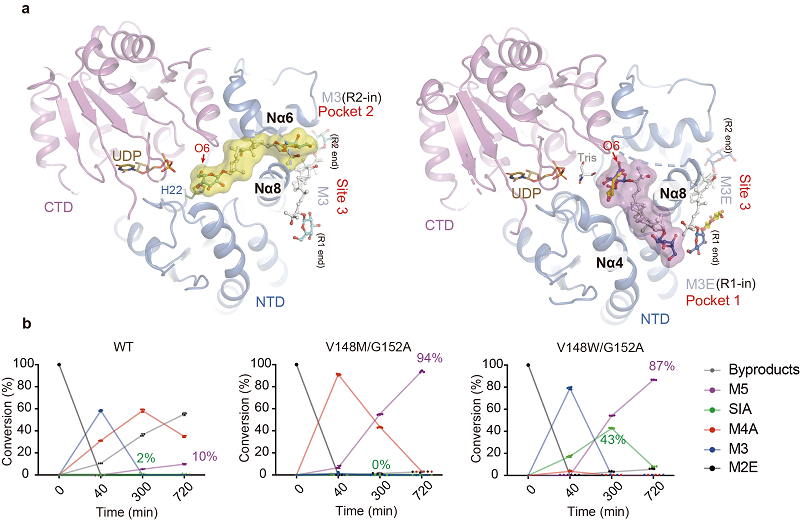
Figure 1. (a) Structural model of SgUGT94-289-3 with mogroside substrates (M3/M3E) bound in two distinct substrate pockets. (b) Conversion rates of various products during the continuous glycosylation reaction catalyzed by SgUGT94-289-3 mutants. (Image by LI Mei's group)
