
2024
RhoBAST, one of the fluorescent light-up RNA aptamers (FLAPs), can bind and activate a contact-quenched type of rhodamine derivative tetramethylrhodamine-dinitroaniline (TMR-DN), a coupled compound of the fluorescent group TMR and the quencher DN. Due to its excellent folding performance, high affinity, rapid ligand exchange, ultra-brightness, outstanding photostability, and other characteristics, RhoBAST exhibits excellent performance in super-resolution imaging of RNA. However, the structural mechanism by which RhoBAST binds and activates TMR-DN is not yet clear.
In a study published in Nature Communications on May 17, a research group led by Prof. FANG Xianyang from the Institute of Biophysics of the Chinese Academy of Sciences (CAS), together with Prof. LI Xing of the Institute of Zoology of CAS, has revealed the structure of RhoBAST and its molecular mechanism of binding and activating TMR-DN by integrating X-ray crystallography, small-angle X-ray scattering, molecular dynamics simulations, and other methods.
The researchers first used X-ray crystallography to resolve the high-resolution structure of the RhoBAST-TMR-DN complex and found that RhoBAST folds into a four-way junction structure, with an overall structure resembling an asymmetric "A" shape.
Small-angle X-ray scattering experiments showed that the folding of RhoBAST does not depend on ligand molecules, and this structural rigidity allows it to avoid photobleaching problems by rapidly exchanging with fresh ligands in the solution, thereby exhibiting excellent optical properties in fluorescence super-resolution imaging.
The researchers used RhoBAST to characterize the conformational space of TMR-DN in both the free and bound states with RhoBAST using enhanced sampling molecular dynamics simulations. They found that, whether in the free or bound state, TMR-DN displays highly dynamic conformational features, with the contact-but-not-attached conformation being predominant.
By integrating multiple research methods, this work elucidated the structure of RhoBAST and its mechanism of binding and activation of the contact-quenched type of fluorophore TMR-DN, and further provides mechanistic insights for the rational design and optimization of this important FLAP system by comparing the structures and binding modes of related FLAPs.
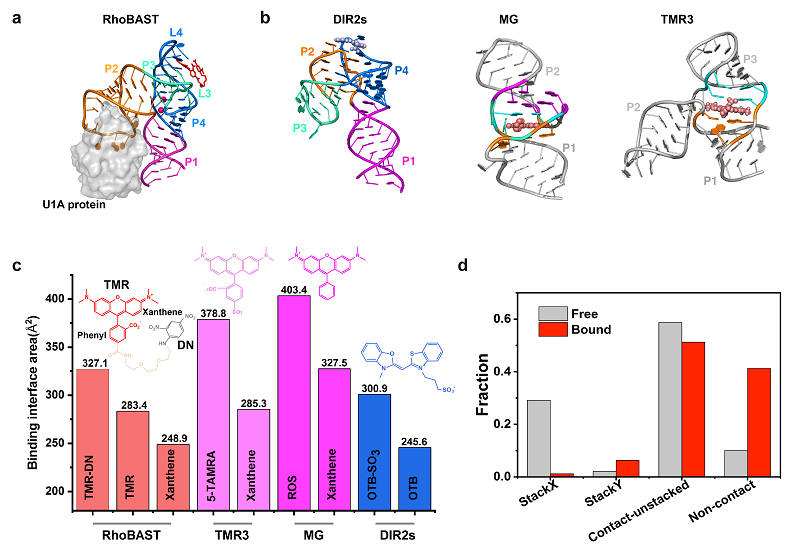
a. A schematic diagram of the crystal structure of the RHOBAST-TMR-DN complex; b-c. Comparison of structures and binding areas of other representative fluorescent RNA aptamer complexes; d. Statistical analysis of different conformations before and after the binding of TMR-DN to RhoBAST. (Image by FANG Xianyang's group)
