
2023
According to a study published in Renewables, a research team led by Prof. HU Linhua from the Hefei Institutes of Physical Science of the Chinese Academy of Sciences has improved the performance of metal ion batteries by enhancing the electrical conductivity and ionic mobility of electrode materials.
Anatase titanium dioxide (TiO2) has attracted great interest as a promising anode for lithium-ion batteries due to its unique properties such as stability, low cost, environmental friendliness and safety. However, as a semiconductor, the intrinsically low ionic and electrical conductivity of TiO2 leads to limited capacity and inferior rate capability and cycling performance, which seriously hinders its practical applications.
In this study, the researchers investigated the properties of oxygen vacancies and carbon co-doped O2 hollow spheres (HS-TiO2) and compared them with fully oxidized white TiO2 hollow spheres (W-TiO2).
They found that the bandgap was smaller when carbon dopant and oxygen vacancies (VO) were added to anatase TiO2.
In addition, the presence of localized electrons resulted in a lower barrier for the movement of Li ions, which accelerated the rate of ion diffusion. This made the oxygen vacancies and carbon co-doped TiO2 hollow sphere (HS-TiO2) more reversible than the fully oxidized TiO2 (W-TiO2) when Li ions were added and taken away.
By changing the duration of a solvothermal reaction, the researchers can control the internal structure of the spheres, making them either solid, porous, or hollow. This adjustment allows them to maintain a consistent shape while changing the internal configuration.
The results of this study pave the way for further exploration and optimization of carbon co-doped TiO2 hollow spheres and other similar materials.

(a-d) Calculated charge difference densities, (e) The total density of states plots, and (f-j) Adsorption energy comparison at the same location site of Li+ of four different location sites of Li+. (Image by LI Zhaoqian)
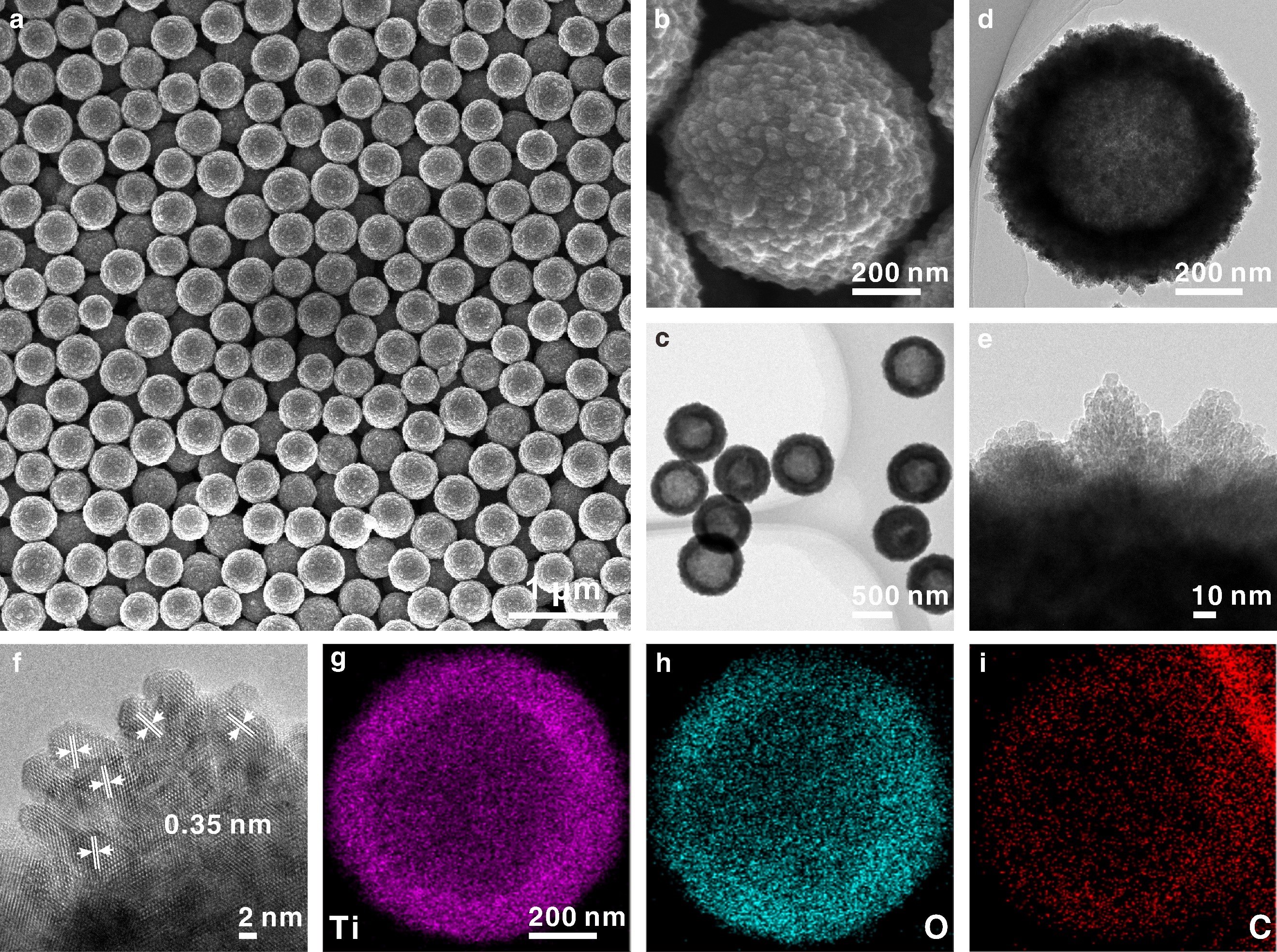
(a, b) Scanning electron microscopy, (c, d) transmission electron microscopy, (e, f) high resolution transmission electron microscopy and (g-i) energy-dispersive system mapping images of HS-TiO2. (Image by LI Zhaoqian)
