Neurons communicate with each other via specialized cellular structures called synapses, formed by axon terminals and opposing dendritic spines, the micron-sized membranous protrusions emanating from dendrites of neighboring neurons. One of the most important features of neuronal cells is synaptic plasticity, the ability of synapses to change their transmission efficacy in response to neural activity evoked by an experience. Consequently, our subsequent emotion, thoughts and behaviors are modified.
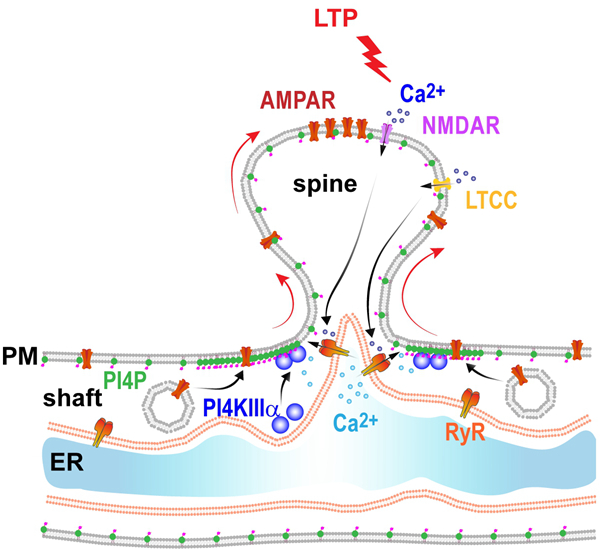
Synaptic plasticity is considered to be the cellular basis of learning and memory. During long-term potentiation (LTP) of hippocampal CA1pyramidal neurons in the brain, a classic form of synaptic plasticity, neuronal activity induces an increase in the number of the AMPA-type glutamate receptors (AMPAR) at the postsynaptic sites located in the plasma membrane of spine heads. How neural activity regulates AMPAR trafficking to the dendritic plasma membrane for synaptic delivery upon LTP induction remains largely unexplored.
A joint research effort uncovered the role of phosphatidylinositol 4-phosphate (PI4P), a minor phospholipid in the lipid bilayer, in activity-dependent AMPAR trafficking and LTP.
In collaboration with Prof. SHUI Guanghou from the Institute of Genetics and Developmental Biology (IGDB) of the Chinese Academy of Sciences and Prof. SHI Yun of Nanjing University, researchers led by Prof. LIU Jiajia at IGDB investigated the dynamic turnover and functional significance of PI4P during synaptic plasticity.
The researchers applied imaging and lipidomic analyses of PI4P in hippocampal neurons undergoing LTP and found that the plasma membrane of hippocampal neurons is enriched in PI4P, and PI4P levels in dendritic plasma membrane undergoes dynamic reversible changes in response to neuronal activity. They then found that PI4P synthesis relies on the activity of the lipid kinase PI4KIIIa.
By pharmacological and genetic manipulations, the researchers further found that plasma membrane PI4P plays an essential role in LTP expression.
Upon LTP induction, rapid increase in cytosolic Ca2+ in the dendritic shaft adjoining potentiated spines induces translocation of PI4KIIIa to the plasma membrane, which catalyzes phosphorylation of phosphatidylinositol to generate more PI4P.
Inhibition of enzyme activity or plasma membrane targeting of PI4KIIIa abolishes activity-triggered exocytosis of AMPAR-carrying vesicles at dendritic plasma membrane, and silencing PI4KIIIa expression in the mouse brain impairs LTP expression and causes severe deficits in long-term memory.
These findings uncover a cellular mechanism for neural activity-dependent plasma membrane trafficking of AMPAR, and demonstrate that PI4KIIIa and PI4P play a vital role in LTP and long-lasting memory.
This research was supported by the National Natural Science Foundation of China, and the Ministry of Science and Technology of China, etc.
Activity-dependent PI4P synthesis promotes AMPAR exocytosis and long-term synaptic potentiation. (Image by IGDB)