
Chinese researchers developed a new nano-alloy material to improve performance of fuel cells.
A fuel cell is a kind of device similar to a power station, which generates electricity from fuel and an oxidant. And it is commonly used in many fields as clean energy.
Among the various clean fuel cells, direct methanol fuel cells (DMFCs) are considered ideal for energy conversion, due to their excellent features of low cost, high theoretical energy density, facile transportation, and good renewable fuel source.
Currently, platinum (Pt) and Pt-based nanostructures are the widely investigated and most efficient catalysts for catalyzing the methanol oxidation reaction (MOR). However, Pt is a kind of metal with low abundance but high costs. Furthermore, most traditional Pt-based catalysts are vulnerable to surface poison, separation, etc., during electrocatalysis.
These side effects decreased their catalytic activity to a large extend.
Given, the research team led by Prof. LI Yue in Institute of solid State Physics (ISSP), Hefei Institutes of Physical Science developed a novel laser-irradiation route to prepare monodispersed spongy AuAgPt ternary alloy nanospheres (spongy AuAgPt NSs).
Owing to the high density of active sites and synergistic effect of Au, Ag, and Pt, the as-prepared spongy AuAgPt NSs exhibited superior catalytic activity toward MOR. And they also showed outstanding operation stability for MOR after long-term cycles.
In summary, this work offers a new efficient strategy for the rational design of 3D spongy electrocatalysts with highly active and stable components for promising applications in biosensing, electrocatalysis, energy conversion, etc.
The findings have been published online in Journal of Materials Chemistry A.
This investigation was supported by the National Key Research and Development Program of China, the Natural Science Foundation of China, the Major Program of Development Foundation of Hefei Center for Physical Science and Technology, the Cross-disciplinary Collaborative Teams Program in CAS, and the CAS/SAFEA International Partnership Program for Creative Research Teams.
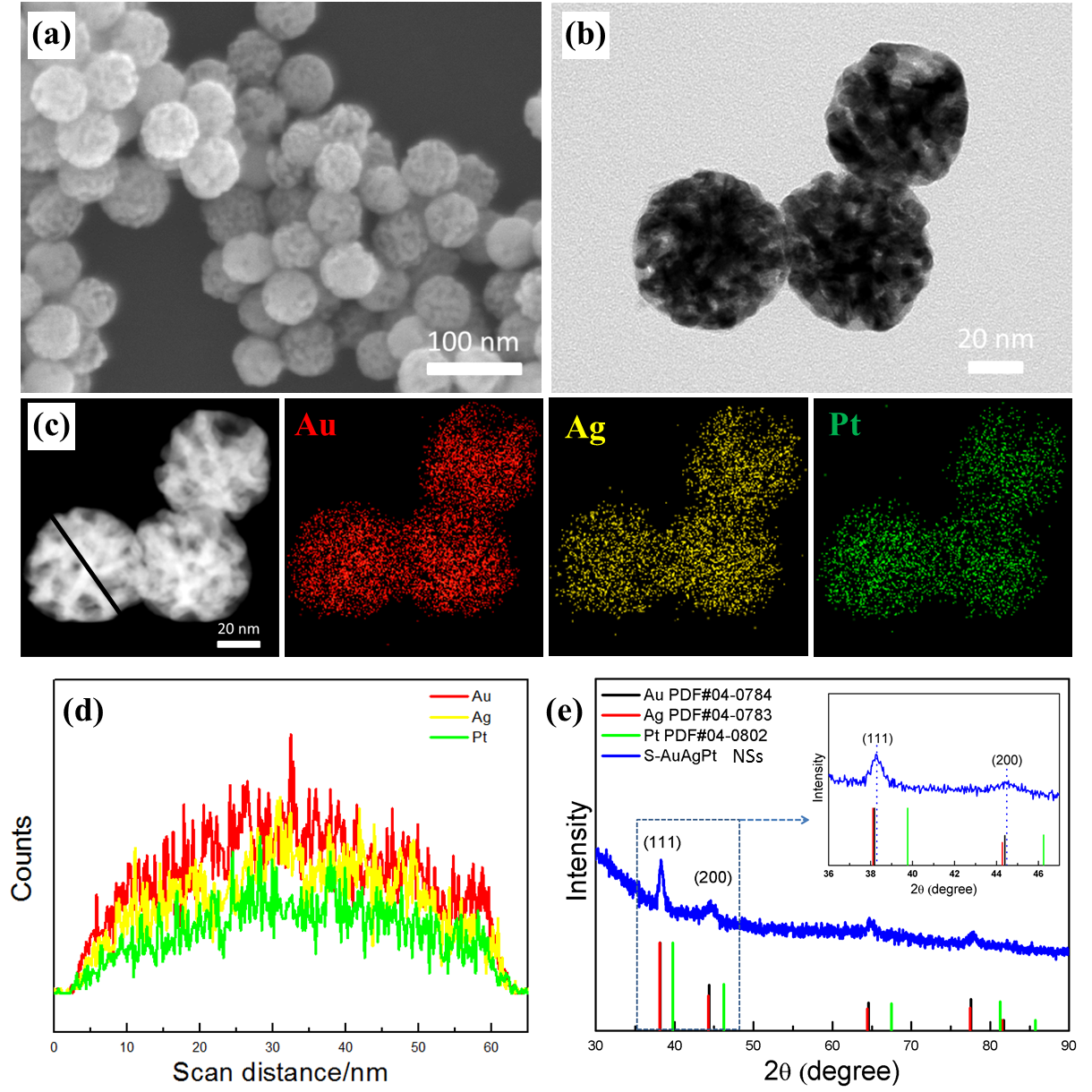
Figure 1. Typical FESEM (a) and TEM (b) images of spongy AuAgPt NSs achieved by a selective chemical dealloying approach using the alloyed AuAgPt NSs as the sacrificial materials, which were obtained through laser irradiating Au@AgPt yolk-shell NCs for 90 s with 532 nm laser beam (laser fluence: 7.67 mJ/cm2). (c) HAADF-STEM image and EDS elemental mapping of ternary spongy AuAgPt NSs. (d) Line scanning profile of single spongy AuAgPt NS marked by black line in (c). (e) PXRD pattern of spongy AuAgPt NSs. (Image by ZHANG Tao)
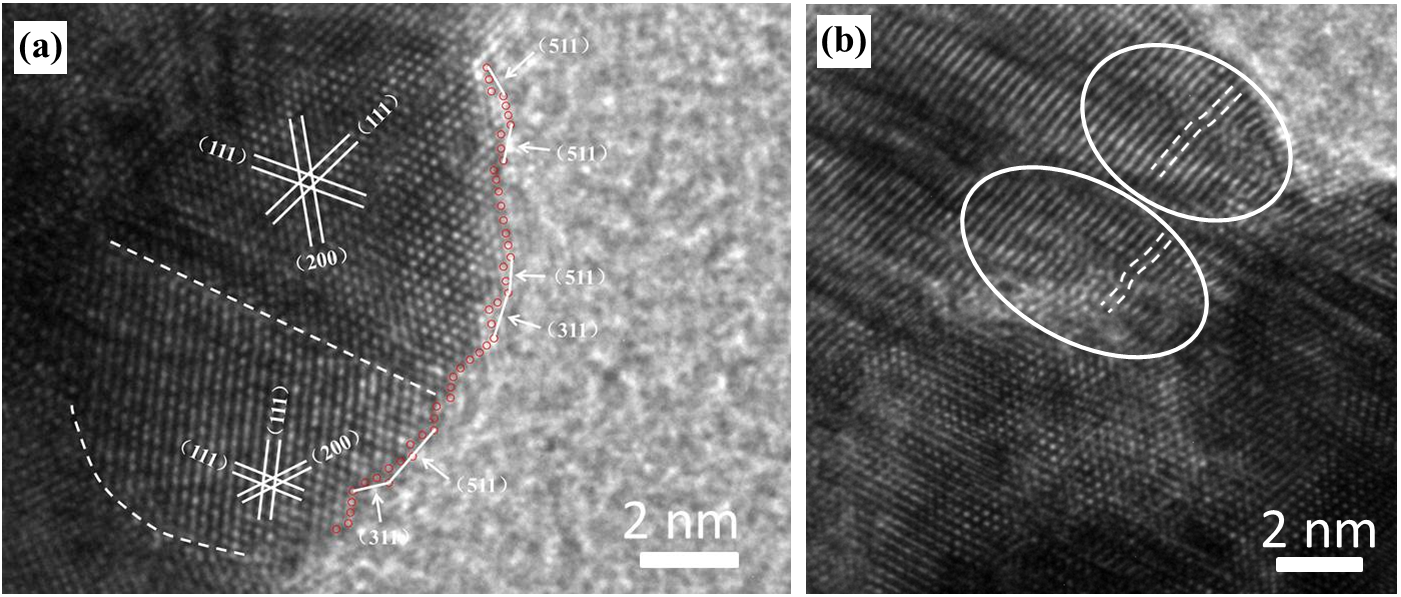
Figure 2. HRTEM images of spongy AuAgPt NS. (a) The grain boundaries marked by white dashed lines and high-index facets indicated by white arrows. (b) Lattice distortion marked by white dashed lines and circles. (Image by ZHANG Tao)

Figure 3. Electrochemistry and MOR performances of the spongy AuAgPt NSs, solid AuAgPt NSs, and commercial Pt black. (a) CVs of the spongy AuAgPt NSs, solid AuAgPt NSs, and commercial Pt black in N2-saturated 0.1 M HClO4 solution at a scan rate of 50 mV/s. (b) MOR curves of these three different catalysts operated in 0.1 M HClO4 + 0.2 M methanol solution at a scan rate of 50 mV/s. (c) Normalized activities of these three different catalysts. (d) Durability comparison of the spongy AuAgPt NSs, solid AuAgPt NSs, and commercial Pt black. (Image by ZHANG Tao)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

