
Coesite is the densest tetrahedrally coordinated crystalline phase of silica, which is the most stable phase at 2GPa. It is classified as a prototype material for pressure-induced amorphization after a group of scientists published their experimental work that coesite was completely amorphized at 34 GPa in 1988.
However, 26 years later, two experimental works shew respectively that coesite was not amorphized at least up to ~51 GPa and transformed into a high-pressure octahedral (HPO) phase.
These experimental works might be reconciled by explanation of different phase transition pathways. Even so, it is urgent to clarify these transition pathways and the phase transition mechanisms.
A recent study conducted by Prof. LIU Changsong's team in Institute of Solid State Physics, Hefei Institutes of Physical Science revealed, with their coworkers abroad, two pressure-induced amorphization pathways of which one could lead to HPO phase for coesite based on classical molecular dynamic simulations with an ab initio parameterized potential.
This collective work, entitled "Multiple pathways in pressure-induced phase transition of coesite", was published in Proceedings of the National Academy of Sciences of USA (or PNAS).
The internationally joint team found that at ambient temperature, coesite transforms into P21/c(I) phase (coesite II is named by Cernok et al.) at 24GPa, and becomes amorphous A(1) at 36 GPa following the first pathway.
They also revealed that coesite transforms into triclinic P-1(II-1) phase at 24GPa, then into P-1(II-2) phase at 28GPa and into amorphous phase A(2) at 48 GPa following the second pathway. These two high-pressure amorphous phases can be described by a mixture of face-centered cubic and hexagonal close-packed oxygen sublattices.
Furthermore, the P-1(II-1) phase transforms into another triclinic P-1(III) phase under good hydrostatic pressure at 34GPa and into a low-symmetry HPO phase P1(2) at 50GPa following the third pathway.
This new HPO phase P1(2) has hexagonal close-packed oxygen sublattice, which is formed through a continuous rearrangement of the oxygen sublattice. The P1(2) phase has 64 SiO2 units per crystalline cell, where only three Si atoms are mismatched compared with α-PbO2 type HPO phase.
The calculated enthalpies of intermediate phases suggest that it is easy for coesite to follow the first path to be amorphized, and far more difficult to follow the third one to transform into the HPO phase. The phase transition behaviors of coesite under high pressure are summarized in the lower figure.
The work well explaines the discrepancies of experimental results of coesite under different compression conditions, moving one step further toward the true understanding of various structural transition mechanisms of a complex material system under high pressure.
The work was financially supported by National Natural Science Foundation of China, and Youth Innovation Promotion Association of the Chinese Academy of Sciences, and the Brazilian Ministry of Science and Technology for collaborative researches between China and Brazil.
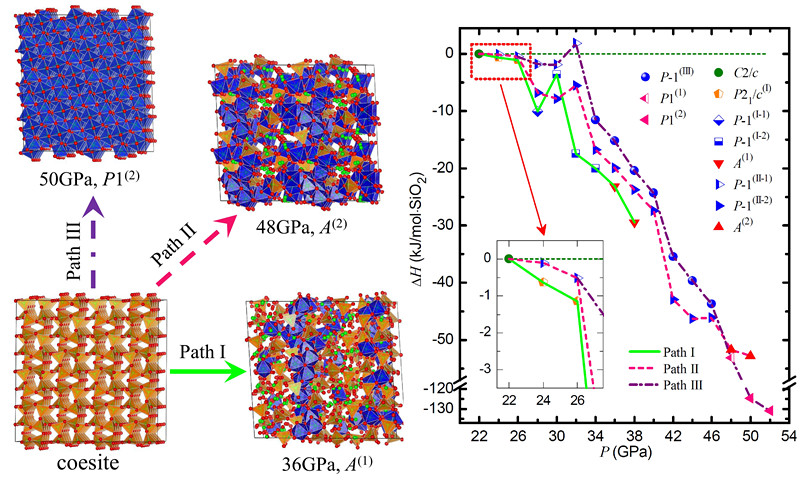
Schematic diagram of three phase transition pathways of coesite. Red and green balls respectively represent O and Si atoms; 4 and 6 coordinated Si atoms are respectively enclosed in orange tetrahedra and blue octahedra. Lower left graph: Relative enthalpy H of different phases along three phase transition pathways. The integer in parentheses behind each phase denotes the number of SiO2 units in the unit cell. Note: Since coesite changes into P21/c(I) or P-1(II-1) phase at 24GPa, the equation of state of coesite is extrapolated to higher pressure using the 3rd order Birch-Murnaghan equation, whose enthalpy is used as reference from 22 to 52GPa. (Image by LIU Wei)

86-10-68597521 (day)
86-10-68597289 (night)

86-10-68511095 (day)
86-10-68512458 (night)

cas_en@cas.cn

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

