
Many proteins in key biological processes are metalloenzymes. Metalloenzymes can achieve high activity under mild conditions utilizing a limited number of physiologically available metal ions and metal-containing cofactors. Rational design of metalloenzymes by realizing the function of a native metalloenzyme in a model protein enables elucidation of structural and mechanistic features responsible for the high activity. It also facilitates the design of cost-effective and efficient catalysts. However, the designed enzymes usually have activities far below those of the native enzymes, which is a major challenge for metalloenzyme design.
Heme-copper oxidases are terminal oxidases of respiratory chain that catalyze the reduction of oxygen to water. These enzymes are more efficient than current platinum-based oxygen reduction catalyst. In a new study published in Journal of the American Chemical Society, WANG Jiangyun’s Group from Institute of Biophysics (IBP) of Chinese Academy of Sciences, in collaboration with LU Yi from University of Illinois at Urbana-Champaign, increased the activity of rationally designed oxidase to the level of native enzymes.
Based on previous work which designs a myoglobin-based oxidase, this study utilized NADH-cytochrome b5 reductase and cytochrome b5 as the electron donor of the myoglobin-based oxidase. When three negative-charged amino acids were replaced by positive-charged lysine, the electron transfer rate between the artificial oxidase and cytochrome b5 was increased by more than 400-fold due to increased electrostatic interaction. After introduction of electron transfer pathway composed of cytochrome b5 reductase, cytochrome b5 and the myoglobin-based oxidase, the oxygen reduction rate was increased from 0.3 s-1 to 52 s-1, achieving the rate of native heme-copper oxidase (50 s-1).
This study confirmed the importance of rapid electron transfer in the reaction of oxidase. It provided new method for designing other metalloenzymes and made it possible to engineer metalloenzymes for biochemical and biotechnology applications such as more efficient ORR catalysts for biofuel cells.
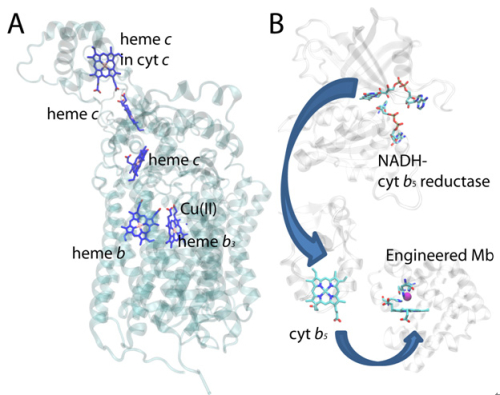
Figure: Electron transfer pathway in a heme-copper oxidase (A) and designed oxidasein this study (B). (Image by WANG Jiangyun’s Group)

86-10-68597521 (day)
86-10-68597289 (night)

86-10-68511095 (day)
86-10-68512458 (night)

cas_en@cas.cn

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

