
The research team led by Prof. YU Shuhong at the University of Science of Technology of China (USTC) of Chinese Academy of Sciences has discovered a highly stable and active electrocatalyst for producing hydrogen from acidic water, even approaching the performance of noble-metal platinum that is an important step to realize the use of water as an energy source in our future society economically. This new electrocatalyst constructed by chemically combining two transitional metal dichalcogenides enables unexpected fast rate for making hydrogen and could be a very promising alternative to platinum.
Cheap and efficient water reduction is an important piece of the big picture of electrochemical energy conversion. However, without a catalyst, water splitting is both slow and inefficient. The most successful catalysts for reducing water so far are platinum-based materials, which are both scare and expensive.
The researchers demonstrated that molybdenum disulfide/cobalt diselenide hybrid can catalyze the water reduction under conditions that approach to the platinum catalyst. By using only cheap elements, the new hybrid catalyst can exhibit ultrafast hydrogen evolution kinetics with onset potential of -11 mV, Tafel slope of 36 mV per decade and exchange current density up to 7.3 × 10-2 mA cm-2. Such a material is still extremely robust after a long-term of operation. These merits suggested the promises for implementing this catalyst into realistic hydrogen evolution electrode.
Collaborating with Prof. LI Jun’s group at Tsinghua University, the team found that the Tafel-step-determined Volmer-Tafel mechanism takes effect in this new hybrid catalyst – a result of electrocatalytic synergistic effects between molybdenum disulfide and cobalt diselenide. The present study may provide a cheaper and more efficient way to make hydrogen to be used for cleaner and more sustainable energy production.
This research project is sponsored by the National Basic Research Program of China, the National Natural Science Foundation of China, the Chinese Academy of Sciences, and the Collaborative Innovation Center of Suzhou Nano Science and Technology.
The work has been published in Nature Communications entitled “An efficient molybdenum disulfide/cobalt diselenide hybrid catalyst for electrochemical hydrogen generation”.
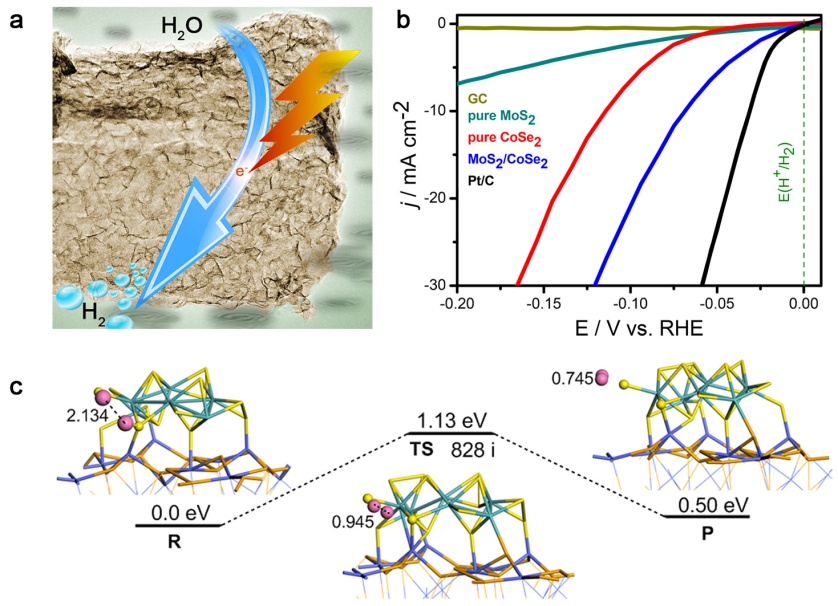
Figure: Schematic of MoS2/CoSe2 hybrid (a), HER polarization curves (b) and HER mechanism (c). (Image by USTC)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

