
Using cryo-electron microscopy, an international group of scientists has solved the atomic structure of the human aichi virus (AiV), a rather unusual but poorly characterized picornavirus that is very common and can cause severe gastroenteritis in children.
Acute viral gastroenteritis is a leading cause of morbidity worldwide and an important cause of death in children less than five years old, especially in developing countries. AiV infects humans, usually subclinically, but can lead to acute gastroenteritis. The seroprevalence of AiV is approximately 60% in children less than 10 years old and reaches 90% later in life. Although AiV is considered a potential global public health threat, there is no available vaccine or effective antiviral treatment.
The identification of the structure of human AiV could be an important step towards a better understanding of how this poorly characterized family of viruses enters host cells and evolves, and could pave the way for new virus treatments.
Professor RAO Zihe and Professor WANG Xiangxi of the Institute of Biophysics (IBP) of the Chinese Academy of Sciences, in cooperation with Professor David Stuart of Oxford University and colleagues, applied cryo-electron microscopy to determine the 3.68 angstrom structure of this elusive virus. AiV has a number of distinctive structural features and seems to occupy a position between the enteroviruses and other picornaviruses, using an uncoating mechanism, yet to be determined, but different from that of enteroviruses.
In their research, a highly exposed polyproline helix at the C-terminus of VP1 was demonstrated to act as a recognition motif for binding to the enteric receptor. This would imply a mode of engagement with the host cell unlike others described for picornaviruses. In addition, the researchers found the interactions between pentamers determine viral particle stability. Enhancing the interactions between pentamers would be a target for the rational design of a picornavirus vaccine to improve its stability.
The research work, entitled Structure of Human Aichi Virus and Implications for Receptor Binding, was published online in the journal Nature Microbiology on September 5, 2016. This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences, the Ministry of Science and Technology 973 Project and the National Science Foundation.
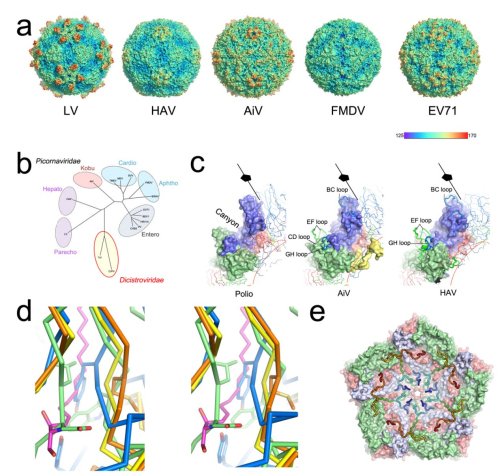
Fig.1 Overall structure of human AiV and structure-based evolution analysis of picornaviruses (Image by IBP)

86-10-68597521 (day)
86-10-68597289 (night)

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

