Headlines
X-ray Laser Sheds Light on Structure of Important Biomolecular Complex
|
Using one of the brightest X-ray lasers in the world, a joint research group involving 28 laboratories and led by Dr. H. Eric XU, a professor from the Shanghai Institute of Materia Medica (SIMM) of the Chinese Academy of Sciences, has determined the structure of a molecular complex that is responsible for regulating vital physiological functions. The new findings provide scientists with a new model for major pharmacological drug targets. The study, Crystal structure of rhodopsin bound to arrestin determined by femtosecond X-ray laser, was published in Nature on July 22.
The 2012 Nobel Prize in Chemistry was shared by Robert J. Lefkowitz and Brian K. Kobilka for their studies on G-protein-coupled receptors (GPCRs). They uncovered a key to physiological information sharing in humans, i.e., how cells sense their environment and transduce external signals into cells through G proteins. However, how GPCRs activate the arrestin signaling pathway, a vital issue in the field of GPCR signaling transduction – an issue scientists had struggled with for many years – is still unanswered.
"Arrestin and G proteins are playing Yin (negative) and Yang (positive) roles in regulating GPCR function,” XU said. Arrestin, as well as other signaling proteins known as G proteins, link up with GPCRs to convey important instructions for many essential physiological functions. Besides the regulation of arrestin in desensitization and internalization of GPCRs, recent research has focused on their function in biased GPCR signaling transduction.
The structural determination of membrane proteins is still a largely unconquered area. It is more challenging to remap the structural image of a large membrane protein complex. For the last decade, a team led by Prof. XU has worked to unravel the structure of a complex made up of arrestin and rhodopsin, which is a prototypical GPCR responsible for light perception and activating visual function.
The most challenging problem in their research was that tiny arrestin-GPCR crystals, which XU’s team had painstakingly produced for years, proved too difficult to study with even the most advanced synchrotron, a more conventional X-ray source. However, with cooperation from different disciplines and by utilizing an X-ray free-electron laser (XFEL) – the brightest laser in the world – scientists obtained a three-dimensional image of rhodopsin-arrestin complex at the atomic level. This represents a much higher resolution than what is possible with older X-ray technology.
This structure reveals the overall architecture of the rhodopsin-arrestin assembly, in which arrestin uses a distinct interaction mode compared with G proteins. This structure provides a basis for understanding GPCR-mediated arrestin-biased signaling and demonstrates the power of X-ray lasers for advancing the frontiers of structural biology.
The discovery solved a world-class scientific question in the field of GPCR signaling transduction, providing a roadmap for developing more selective therapy strategies. “GPCRs are major targets in the development of new therapies and account for almost 40 percent of current drug targets,” said XU.
"In the realm of drug development, a detailed understanding of the structure, interaction and function of each of these groups of proteins is vital to developing effective therapies. The more specific the interaction, the better the drugs tend to work while also lowering the chance of side effects." Accordingly, drugs that only activate either the arrestin or G protein signaling pathway, but not both, can yield better therapeutic benefits with fewer undesirable side effects than non-selective drugs.
"This project represented a significant challenge and was accomplished through the work of a multidisciplinary team from many institutions around the globe,” said XU. “Utilizing the X-ray laser also opens the door for solving future challenging problems.”
"The XU group has put together an important story that provides significant insight into our understanding of G-protein-coupled receptor function,” said Jeffrey Benovic, Ph.D., Thomas Eakins Professor at Thomas Jefferson University. “The rhodopsin-arrestin structure helps to explain the process of desensitization and provides a roadmap for obtaining the structure of additional GPCR complexes.”
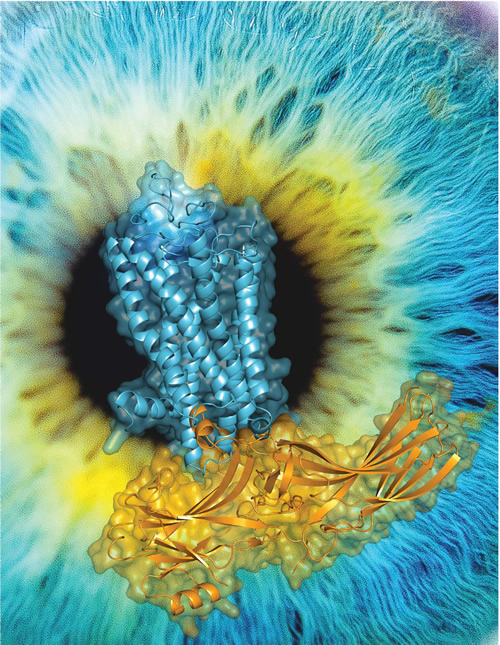
High-resolution 3D structure of Rhodopsin-arrestin complex. Blue, the structure of rhodopsin. Yellow, the structure of arrestin. Rhodopsin is a prototypical GPCR responsible for light perception and visual generation, a process arrestin is involved in. (Image by XU's lab)
Contact