
The immune system protects an organism against a wide variety of pathogens. A unique system exists in prokaryotes and plays an important role in defending against invasive bacteriophages and plasmids. It is the CRISPR/Cas system which is formed by clustered regularly interspaced short palindromic repeats (CRISPR) together with CRISPR-associated (Cas) proteins.
Functions of biomolecules are intensively influenced by their structure. High resolution crystal structure reveals critical insight for understanding complex biological mechanisms.
Many studies had been conducted to determine the structure of CRISPR/Cas system. But due to its complexity, previous effort only obtained low-resolution cryo-electron microscopy structures, which provided limited information to explain the system’s function and mechanism.
Professor WANG Yanli at the Institute of Biophysics and her colleagues recently reported a breakthrough in this field in the top-tier scientific journal Nature.
WANG’s team used Escherichia coli as model organism and studied the I–E subtype CRISPR/Cas system, which includes eleven subunits from five Cas proteins (CasA1B2C6D1E1) assemble along a CRISPR RNA (crRNA). They determined the 3.05 Å crystal structure of the 405-kilodalton Escherichia coli Cascade complex. (Figure)
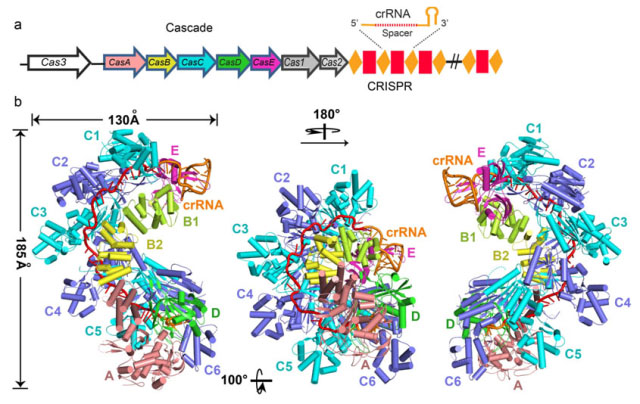
Figure: Crystal structure of the Cascade complex from E. coli. a, The I–E subtype CRISPR system in E. coli (K12) consists of eight Cas proteins and CRISPR locus. b, Overall structure of the Cascade complex.(Image by WANG Yanli et.al)
Their structural analysis revealed the sophisticated protein interaction network in the Cascade organization. They found that the Cascade complex shapes like a sea horse in which the bound 61-nucleotide crRNA spans the entire 11-protein subunit-containing complex. Scientists demonstrated how the two ends of the crRNA are anchored, and clarified how the spacer fragment is positioned in the continuous groove on the concave surface of CasC1–6. They also explained the mechanism how the Asp-Arg/Lys-Trp triad mediates the assembling of CasA and CasB to the six-CasC helix. Their structure elucidates why the seed sequence, with its outwards-directed bases, has a critical role in target DNA recognition.
This study provides novel molecular details of how the Cascade complex functions through protein–protein and protein–RNA alignments and interactions to mediate RNA-guided immune surveillance.

86-10-68597521 (day)
86-10-68597289 (night)

86-10-68511095 (day)
86-10-68512458 (night)

cas_en@cas.cn

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

