
The ERK (extracellular signal-regulated kinase) pathway plays a critical role in the vital processes of living cells such as proliferation and differentiation. Recently, CAS scientists in Shanghai have discovered a novel mechanism of spatial regulation on ERK pathway. The result will be published in the 4 September issue of the Proceedings of National Academy of Sciences (PNAS).
The ERK signaling pathway is under strict control in healthy cells. Difference in factors such as cellular types, types of stimuli, as well as the strength and duration of stimulation could lead to various ERK signals, causing distinctive biological functions. Anomalies in the ERK signal-regulated mechanism may invoke the emergence of cancers. Recent studies have indicated that RAS and MEK, two major components of the ERK pathway, are under the control of spatial regulation. However, scientists were not clear whether or not Raf, a protein that bridges RAS and MEK in ERK pathway, is also under similar control.
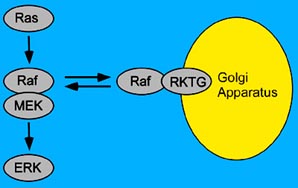
A research team led by Prof. CHEN Yan at the Institute for Nutritional Sciences, the CAS Shanghai Institutes for Biological Sciences, has found a new negative regulatory protein for ERK pathway. Their study indicates that a new protein, named as RKTG (Raf kinase trapping to Golgi), is implicated in the spatial regulation of Raf kinase.
The scientists found that RKTG is a close homologue of adiponectin receptors. Adiponectin plays an important role in the regulation of energy and glucose metabolism. Different from adiponectin receptors, RKTG is specifically localized at the Golgi apparatus, a major subcellular organelle that packages large molecules for transport within the cell. Through interaction with Raf-1, RKTG can sequester intracellular Raf-1 to the Golgi apparatus, whereby interfering with the interaction of Raf-1 with its upstream and downstream proteins. In addition, RKTG reduces the activation of Raf-1 kinase and blocks the signal transduction fo RAS to its downstream targets, finally leading to the inhibition of the ERK pathway. This scheme of regulation is further supported by their study with the mouse. When RKTG is deleted, enhanced ERK activation is observed in many tissues. When activated by growth factors, the phosphorylation of ERK increased and prolonged in cells isolated from the RKTG knockout mouse.
This discovery is important in revealing a novel mode of regulation on Raf kinase. It not only demonstrates that Raf-1 is under spatial regulation, but also uncovers a new mechanism in which the ERK signaling pathway is modulated by subcellular compartmentation at the Golgi apparatus. Considering that the ERK pathway plays a pivotal role in cancer formation, this study will be of great help in further understanding the molecular mechanism underlying excessive proliferation of malignant cells.

86-10-68597521 (day)
86-10-68597289 (night)

86-10-68511095 (day)
86-10-68512458 (night)

cas_en@cas.cn

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

