
Two-dimensional (2D) MoS2 has shown good catalytic activity for hydrogen evolution reaction (HER) and is considered as a potential alternative to the precious platinum-based catalysts in acidic medium.
However, only the edge S sites of pure MoS2 are catalytically active for HER and the vast amount of S sites in the basal plane are quite inert and not sufficiently utilized. Besides, the low stability of edges also limits HER performance of MoS2.
Therefore, selectively activating the inert basal plane combined with enriching and stabilizing edges can increase active sites of MoS2 for HER. It is still highly challenging owing to the difficulty in balancing activity and stability.
Recently, Prof. DENG Dehui from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) reported a MoS2 nanofoam catalyst with co-confined Se in the surface and Co in the inner layer of the tri-atomic-layer structure of MoS2. It exhibited high catalytic activity and durability for acidic HER at a large current density of 1000 mA/cm2.
The study was published in Nature Communications on July 3. It opens up a new prospect of tailoring the catalytic performance of MoS2 toward large-scale HER applications through confining multi-elements in different atomic layers.
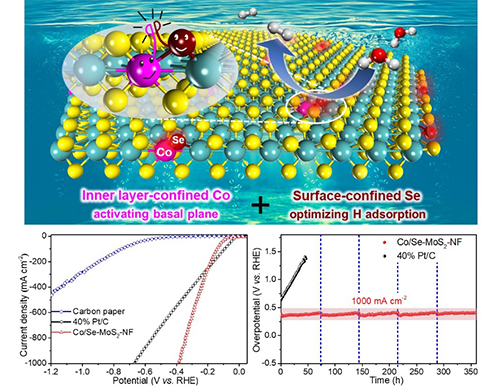
High-performance hydrogen evolution reaction over the Co/Se-codoped MoS2 (Image by DICP)
The scientists previously reported a strategy of confining transition metal atoms into MoS2 lattice to trigger HER activity over S atoms in the inert basal plane.
In this study, they found that co-confining Se in the surface and Co in the inner layer of the MoS2 could realize simultaneously activation of the basal plane, optimization of the hydrogen adsorption activity, and stabilization of the structure.
"Such catalyst exhibited an ultra-high HER activity in acidic medium," said Prof. DENG.
They achieved a much lower overpotential of 382 mV than that of 671 mV over commercial Pt/C catalyst at a large current density of 1000 mA/cm2, and it stably maintained for 360 hours without decay. The activity surpassed those of all previously reported heteroatom-doped MoS2.
Density functional theory calculations demonstrated that inner layer-confined Co atoms stimulated neighbouring S atoms while surface-confined Se atoms stabilized the structure, which cooperatively enabled the massive generation of both in-plane and edge active sites with optimized hydrogen adsorption activity.
The study was supported by the National Key R&D Program of China, the National Natural Science Foundation of China, the Key Research Program of Frontier Sciences of the Chinese Academy of Sciences, the DNL Cooperation Fund, CAS, and Collaborative Innovation Center of Chemistry for Energy Materials (2011. iChEM).

86-10-68597521 (day)
86-10-68597289 (night)

86-10-68511095 (day)
86-10-68512458 (night)

cas_en@cas.cn

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

