
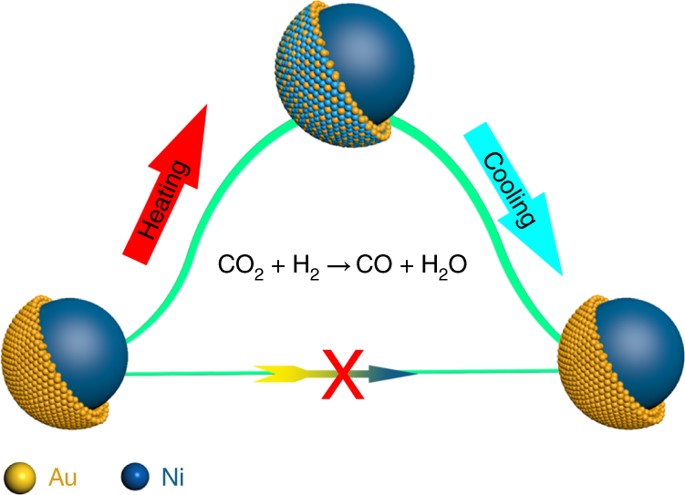
The high catalytic performance of core–shell nanoparticles (NP) is usually attributed to the synergy of distinct geometric and electronic structures. Although the general assumption is that core–shell NPs maintain their configuration under working conditions, it remains unclear whether they maintain their structure throughout a reaction.
Scientists recently reported a different working mechanism and demonstrated that the core–shell structure may not exist under a specific set of conditions, which overturns the conventional understanding.
The results appeared in a study conducted by a team from the Shanghai Advanced Research Institute (SARI) of the Chinese Academy of Sciences (CAS) along with other collaborators in the latest issue of Nature Catalysis entitled “Reversible loss of core–shell structure for Ni–Au bimetallic nanoparticles during CO2 hydrogenation".
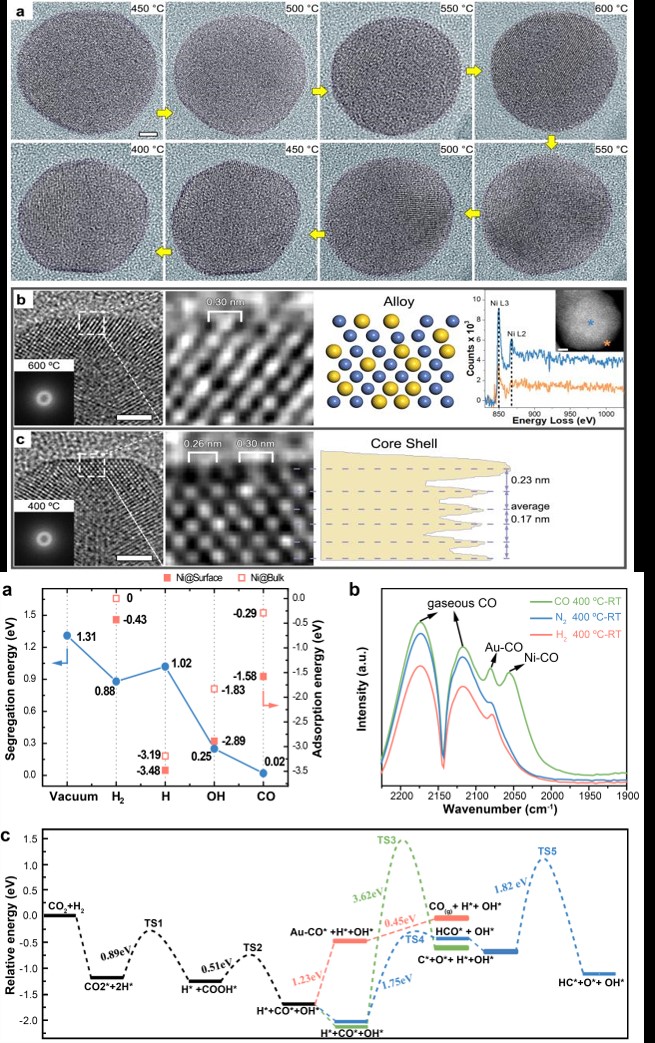
The scientists used environmental transmission electron microscopy (ETEM) to directly visualize the dynamic process at atomic level, coupled with multiple state-of-the-art in situ techniques, including synchrotron X-ray absorption spectroscopy, infrared spectroscopy and theoretical simulations, to precisely analyze the imaging conditions of over 3,000 high-resolution transmission electron microscopy (TEM) images.
By tracing the real-time changes of the surface atomic structure during the entire reaction process, the result exhibits a highly selective CO production in CO2 hydrogenation, features an intact ultrathin Au shell over the Ni core before and after the reaction. However, the catalytic performance could not be attributed to the Au shell surface, but rather to the formation of a transient reconstructed alloy surface, promoted by CO adsorption during the reaction.
Density functional theory (DFT) calculations also confirmed that it is the kinetically alloyed surface, rather than the ultrathin Au shell surface, that is catalytically active during the highly selective reverse water gas shift reaction.
The discovery of such a reversible transformation urges us to reconsider the reaction mechanism beyond the stationary model, and may have important implications not only for core–shell nanoparticles, but also for other well-defined nanocatalysts.

86-10-68597521 (day)
86-10-68597289 (night)

86-10-68511095 (day)
86-10-68512458 (night)

cas_en@cas.cn

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

