
Recently, various internal metabolic processes have emerged as new mechanisms to regulate function of immune cells, other than the traditional invading pathogens. As an essential process of cellular homeostasis, iron metabolism has been associated with many inflammatory conditions.
Abnormal accumulation of iron is frequently observed in the brain of patients with neuroinflammatory diseases or neurodegenerative diseases for more than a decade, yet its contribution to the disease pathogenesis is still poorly understood.
Increased iron levels were observed in the earliest onset of some of these diseases, suggesting that they are a potential cause rather than a consequence of neuronal damages.
A research team led by Dr. CHANG Xing from Shanghai Institute of Nutrition and Health, Chinese Academy of Sciences demonstrated for the first time that iron could rapidly and actively induce inflammatory responses, in contrast to the previous understanding that it only passively participated in oxidative stress.
In this study, researchers showed that iron post-transcriptionally promoted cytokine production in autoreactive T cells via a Poly rC binding protein 1 (PCBP1)-dependent mechanism.
Either intracellular iron depletion or PCBP1 deficiency in autoreactive T cells resulted in shortened mRNA half-life and abolished cytokine production both in vitro and in experimental autoimmune encephalomyelitis (EAE).
GM-CSF, a proinflammatory cytokine indispensable for T cell-mediated encephalomyelitis, was most sensitive to either iron depletion or PCBP1 deficiency in T cells.
Strikingly, two structurally distinct iron chelators both induce caspase-mediated proteolysis of PCBP1 protein and block its activity in promoting GM-CSF production, indicating PCBP1 acted as an iron sensor to mediate this metabolic signaling to modulate cellular transcriptomes.
This study identifies PCBP1 as the first RNA-binding protein to be required for the pathogenesis of T cell-induced autoimmune disease, and establishes a novel mechanistic link between post-transcriptional gene regulation and iron metabolism.
The result suggests that iron deposition can directly promote inflammation and precipitate inflammatory diseases. The pathway identified here may offer new targets for the treatment of inflammatory diseases, such as interference in the Iron-Caspase-PCBP1 pathway aiming at blocking the generation of GM-CSF-expressing pathogenic T cells.
This work has been published online ahead of printing in Immunity tilted "Iron drives T helper cells pathogenicity by promoting RNA-binding protein PCBP1- mediated proinflammatory cytokine production".
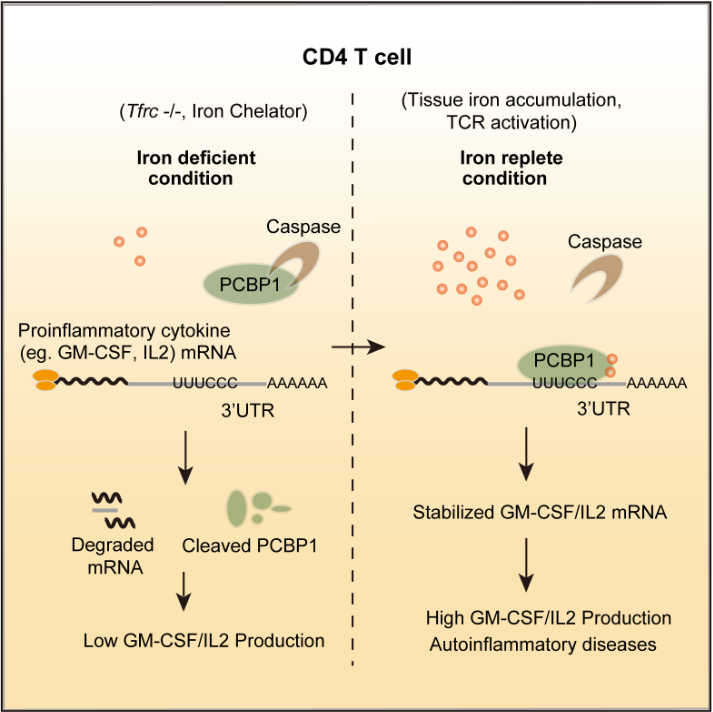
Model of "Iron-PCBP1-Cytokine" in T cells (Image by Prof. CHANG Xing's Group)

86-10-68597521 (day)
86-10-68597289 (night)

86-10-68511095 (day)
86-10-68512458 (night)

cas_en@cas.cn

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

