
A research group led by Prof. HAN Keli from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences reported an advanced review paper entitled "Unraveling the Detailed Mechanism of Excited-State Proton Transfer" in Account of Chemical Research.
This review briefly summarized the research progress on the mechanism of excited-state proton transfer (ESPT) from HAN's group since 2009, discussed the advantages and disadvantages of different theoretical methods on describing the potential energy surface of the ESPT, and provided an outlook of the opportunity and challenge ahead of the research area.
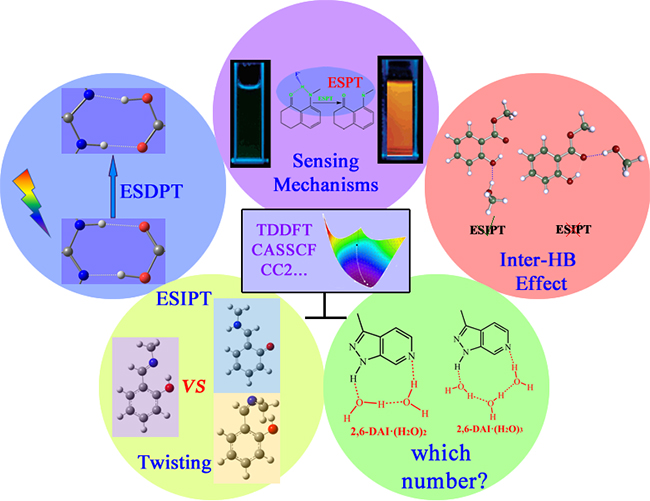
The cases where the theoretical studies are of great importance and indispensable. (Image by ZHOU Panwang)
ESPT is one of the most fundamental processes that plays vital roles in biological systems, and an increasing number of new chromophores based on the ESPT have been developed. Therefore, investigating the mechanism of ESPT at the atomic and molecular level is of great biological significance, and can provide guidance for researchers to design new ESPT chemosensors.
In 2009, HAN's group firstly investigated the excited-state double proton transfer (ESDPT) between 2-aminopyridine (2-AP) and the acetic acid by using the time-dependent density functional theory (Phys. Chem. Chem. Phys.). They demonstrated that the ESDPT reaction of 2-AP/acid system follows stepwise mechanism.
They theoretically investigated the sensing mechanism of a series of fluorescent probes based on ESPT (WIREs Comput. Mol. Sci.), the effect of intermolecular hydrogen bonding on excited-state intramolecular proton transfer (ESIPT), competition between ESIPT and twisting processes, and the mechanism of solvent-assisted ESPT reaction.
It was found that the theoretical studies on the ESPT systems could provide modifications to the proposed mechanism based on the experimental results and might present a completely new mechanism that was in accordance with the experimental results (J. Phys. Chem. B).
Moreover, the theoretical studies on the ESPT systems may inspire experimental scientists to perform investigations to confirm the proposed mechanism based on the theoretical results (J. Phys. Chem. Lett.).
This work was supported by the National Natural Science Foundation of China, and the Science Challenging Program.

86-10-68597521 (day)
86-10-68597289 (night)

86-10-68511095 (day)
86-10-68512458 (night)

cas_en@cas.cn

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

