
Recently, a research team led by Prof. WANG Feng from Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) found that photo-induced N-oxyl radical, which was confined on metal oxide surface, could selectively achieve the activation of C-H bonds. Their findings were published in J. Am. Chem. Soc..
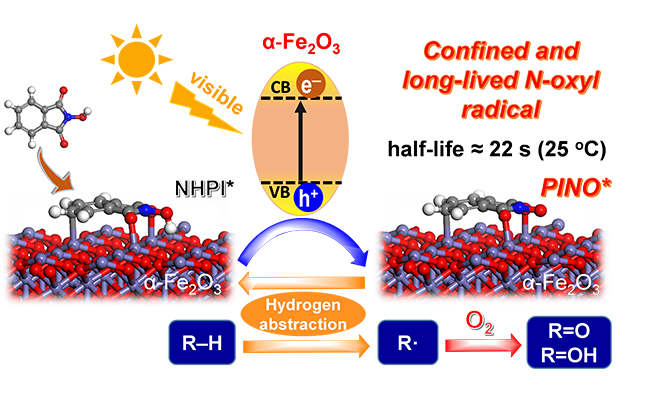
Confined phthalimide-N-oxyl radical (PINO) on α-Fe2O3 surface in the photocatalytic C(sp3)–H activation under visible light. (Image by ZHANG Chaofeng)
The selective oxidation of hydrocarbons to oxygenates has been an important topic in the field of catalysis. One of the representative examples is using N-oxyl radical to firstly abstract the H of C-H bond to a carbon radical, which then activates the O2 at ground state, leading to oxygenated products.
However, a long-time unsolved issue for this approach is that it needs to use radical initiators to generate N-oxyl radical. Furthermore, the free N-oxyl radicals are short-lived with a millisecond scale life time (J. Org. Chem.) and over-reactive, which bring out side reactions during the hydrocarbon oxidation.
Researchers reported a novel strategy of photoinduced surface-confined N-oxyl radical for C-H bonds oxidation. The photo-generated hole on α-Fe2O3 under visible light irradiation oxidized N-hydroxyphthalimide (NHPI) to phthalimide-N-oxyl radical (PINO*). The generated PINO* radical was confined on the oxide surface. This confinement extended the radical half-life time to 22 seconds after turning off the light, which was proved by in-situ electron paramagnetic resonance.
The catalytic system with a long-lived N-oxy radical showed high activity for C-H bonds oxidation, while the α-Fe2O3 alone showed no activity. Therefore, the transformation of NHPI to a confined PINO* not only promoted the separation of hole and the electron of α-Fe2O3, but also transformed the short-lived hole to a long-lived and versatile N-oxyl radical for C-H bonds activation.
The unique generation mechanism of the confined N-oxyl radical may open up a new topic about regulating the properties of functional radicals and offering a new way of utilizing organic radical in selective oxidations.
This work was supported by the Strategic Priority Research Program of CAS, National Natural Science Foundation of China and DICP.

86-10-68597521 (day)
86-10-68597289 (night)

86-10-68511095 (day)
86-10-68512458 (night)

cas_en@cas.cn

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

