
Dienes and polyenes are of high synthetic interest due to their frequent presence in medicinally relevant molecules and natural products. Within this context, transition metal-catalyzed dehydrogenation reactions provide powerful strategies to construct such molecular scaffolds in an efficient manner from simple starting materials.
The research group led by Prof. SU Weiping from Fujian Institute of Research on the Structure of Matter of the Chinese Academy of Sciences (CAS) reported a copper-catalyzed method to access diverse diene, polyene and heterocycles motifs via multiple successive dehydrogenation process. This finding was published in Nature Communications.
This novel approach estabilished a Cu(OAc)2/TEMPO catalytic system for the successive dehydrogenation to afford dienes and polyenes in highly E-seletivity. This protocol is applicable to a broad range of substrate including ketones, aldehydes, alcohols, and a,b-unsaturated diesters.
Besides carbonyl compounds, tetrahydroquinolines and NH-free indolines also underwent successive dehydrogenation to afford the corresponding aromatic N-heterocycles in excellent yields.
Mechanistic studies revealed that this dehydrogenative desaturation-relay proceeded through a multicenter-stabilized radical intermediate.
To identify this, g-TEMPO-substituted enone was isolated and a radical clock experiment was also carried out. All of these results supported that this successive dehydrogenation reaction proceeded through a g-enone radical intermediate.
As an evidence of applications, the successive dehydrogenation strategy was employed to synthesis polyene natural products in synthetic useful yields.
Two insect alarm pheromones, lignarenone B and navenone B, were synthesized in two steps from commercially available materials with synthetic useful yields.
This study displays a powerful method to synthesis valuable dienes and polyene. Furthermore, it also opens a door to expend this series of transformations to functionalize C-H bonds lies in the distal positions from functional groups.
This work was supported by NSFC, the CAS/SAFEA International Partnership Program for Creative Research Teams, Youth Innovation Promotion Association of CAS, the Strategic Priority Research Program and the Key Research Program of CAS.
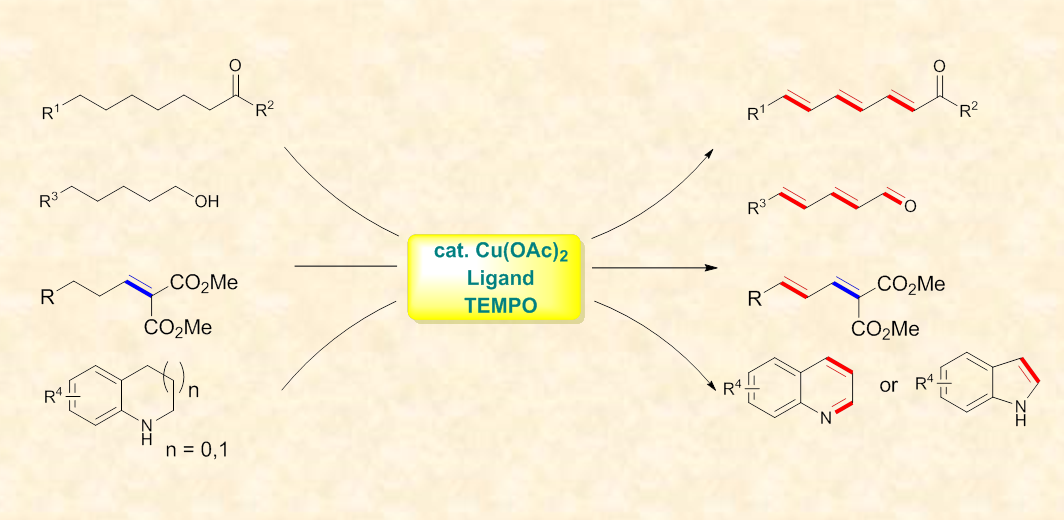
Cu-catalyzed successive dehydrogenation of diverse starting materials (Image by Prof. SU's Group)

86-10-68597521 (day)
86-10-68597289 (night)

86-10-68511095 (day)
86-10-68512458 (night)

cas_en@cas.cn

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

