
Due to the great surface-to-volume ratio, microplastics can sorb and concentrate various types of environmental chemicals from the external phases. Therefore, microplastics are potential carrier for pollutants in the natural environment.
A better knowledge of interactions between polycyclic aromatic hydrocarbons and microplastics is of vital importance for evaluating the possible influence of microplastics on the moving dynamics of these organic chemicals in aquatic environments.
Under the guidance of Prof. WANG Jun, WANG Wenfeng from Wuhan Botanical Garden of the Chinese Academy of Sciences investigated the sorption process of pyrene (Pyr), a frequently encountered toxic pollutant, onto three kinds of microplastics (polyethylene, polystyrene and polyvinylchloride) by comparatively analyzing different sorption kinetic and isotherm models.
The kinetics study revealed that the sorption rates of Pyr onto microplastics were dominated mainly by the intraparticle diffusion, although the pseudo-second-order kinetic model was more appropriate in describing the entire sorption process (R2> 0.99).
The sorption equilibrium data were the best fitted to the Langmuir isotherm (R2> 0.99). The present study could pave the way towards a better understanding of sorption mechanisms of organic pollutants onto microplastics.
Results were published in Chemosphere entitled "Comparative evaluation of sorption kinetics and isotherms of pyrene onto microplastics".
This study was supported by Funding Project of Sino-Africa Joint Research Center, Chinese Academy of Sciences, the Hundred Talents Program of the Chinese Academy of Sciences and Natural Science Foundation of Hubei Province of China.
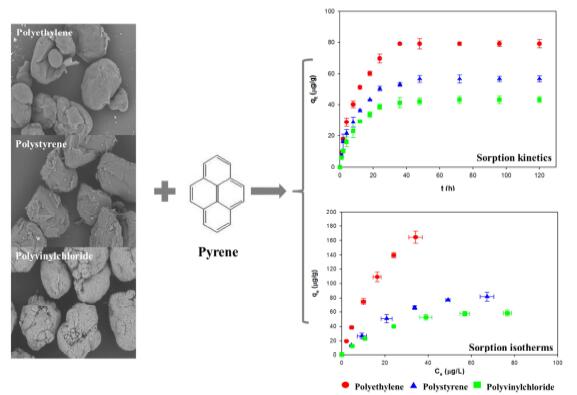
Sorption kinetics and isotherms of pyrene onto microplastics (Image by WANG Wenfeng)

86-10-68597521 (day)
86-10-68597289 (night)

86-10-68511095 (day)
86-10-68512458 (night)

cas_en@cas.cn

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

