
Recently, A team led by WU Zhengyan from Institute of Technical Biology and Agriculture Engineering, Hefei institutes of physical science, developed a nanocomposite to remediate Cr(VI)-contaminated water and soil.
The nanocomposite was synthesized using graphene oxide (GO), polyethylenimine (PEI) and Fe3O4. This research was published in Langmuir with the title of Synthesis of a multifunctional graphene oxide-based magnetic nanocomposite for efficient removal of Cr(VI).
Hexavalent chromium (Cr(VI)), a stable state of chromium, is commonly presented in waste water and soil. It is rather toxic and can cause a lot of diseases including liver damage, pulmonary congestion, severe diarrhea, and skin irritation.
Meanwhile, Trivalent chromium (Cr(III)), another stable state of chromium, displays obviously lower toxicity, carcinogenicity, solubility, and mobility compared with Cr(VI). Therefore, it is crucial to remove Cr(VI) from wastewater and soil or reduce highly toxic Cr(VI) to low toxic Cr(III).
To aim at this, WU’s team incorporated Fe3O4 with GO/PEI to obtain RGO/PEI/Fe3O4 (the optimal one is designated as ORPF).
This magnetic nanocomposite, with functions of high adsorption, reduction, and collectability, could efficiently remove Cr(VI) from water and soil, and control the migration of Cr(VI) in soil.
Therein, the adsorption effect was mainly due to the electrostatic attraction between -NH3+ and Cr(VI), and the reduction from Cr(VI) to Cr(III) was mainly attributed to π electrons of reduced GO. Then Cr(III) could tightly bind to ORPF through the chelation of -NH2 and electrostatic attraction of -COO-.
Moreover, the resulting ORPF/Cr could be collected from water and soil conveniently by a magnet.
This work provides a facile, efficient, and environmentally-friendly approach for the remediation of Cr(VI)-contaminated water and soil.
This research was supported by the National Natural Science Foundation of China, the Youth Innovation Promotion Association of Chinese Academy of Sciences, the Key Program of Chinese Academy of Sciences, and the Science and Technology Service Programs of Chinese Academy of Sciences.
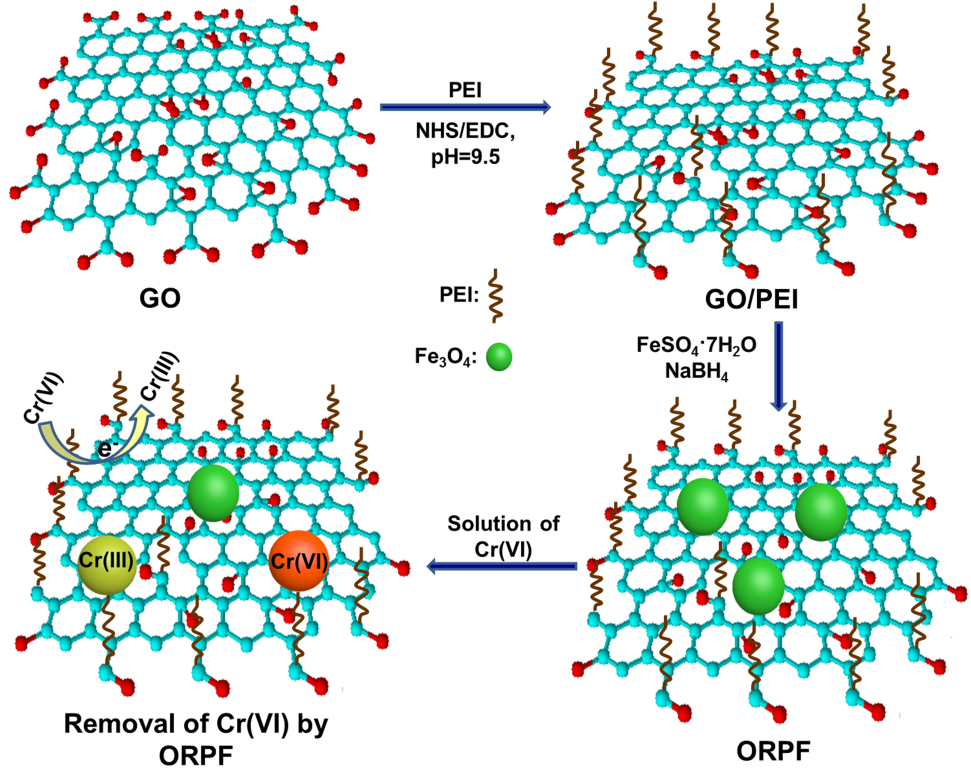
Schematic diagram of the synthesis of ORPF and the mechanism of the removal of Cr(VI). (Image by WANG Dongfang)

86-10-68597521 (day)
86-10-68597289 (night)

86-10-68511095 (day)
86-10-68512458 (night)

cas_en@cas.cn

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

