
CRISPR/Cas systems are widespread RNA-mediated prokaryotic adaptive immune systems providing protection against invading nucleic acids. However, this host defense system has not resulted in the eradication of phages, suggesting that phages have evolved counter strategies to thrive within bacteria despite these mechanisms.
The type I-F CRISPR-Cas system,crRNA assembles with Csy1, Cys2, Csy3 and Csy4 proteins, forming a crRNA-guided surveillance complex (named Csy complex). The Csy complex specifically recognizes the complementary target DNA and recruits Cas3 to degrade the invading DNA.
Previous study shows that the some phages contain a set of atypical genes encoding small phage proteins involved in inactivating the CRISPR/Cas system. AcrF3 interacts with Cas3 protein and blocks its recruitment by Csy complex, thus protects the phage DNA from being degraded by the CRISPR/Cas system.
Recently, WANG Yanli’s group and ZHANG Xinzheng’s group from the Institute of Biophysics (IBP) of Chinese Academy of Sciences (CAS) solved the crystal structure of AcrF3 and cryo-EM structure of AcrF3-Cas3 complex. This study was published online in Cell Research.
In the structure of AcrF3-Cas3 complex, two AcrF3 form a dimer. The AcrF3 dimer binding site of Cas3 is the entrance of the displaced single-stranded DNA associated with Cas3, suggesting that the AcrF3 dimer blocks the access to Cas3 of the non-target DNA. When AcrF3 binds to Cas3, Csy-DNA complex could not recruit Cas3 to degrade phage DNA, resulting in the escape of phages from the immune response.
It was found that the Cas1-Cas2 complex associates with the Cas3 protein during primed acquisition. The degradation products of Cas3 are used by Cas1-Cas2 as precursors for new spacers. AcrF3 inhibits Cas3 recruitment by Csy-dsDNA complex, thus stopping the degradation of phage DNA and generation of the precursor protospacer DNA.
In summary, the AcrF3 dimer overcomes the CRISPR system at both the crRNA interference and the primed acquisition stages. This study provides new insights into the ongoing molecular arms race between viral parasites and the immune systems of their hosts.
The research was funded by grants from the National Natural Science Foundation of China, the Chinese Ministry of Science and Technology, the Strategic Priority Research program of Chinese Academy of Sciences and scholarships from the National Thousand (Young) Talents Program from the Office of Global Experts Recruitment in China.
The X-ray diffraction data were collected at beamlines BL-19U at the Shanghai Synchrotron Radiation Facility. The Cryo-EM data were collected at the Center for Biological Imaging (IBP, CAS).
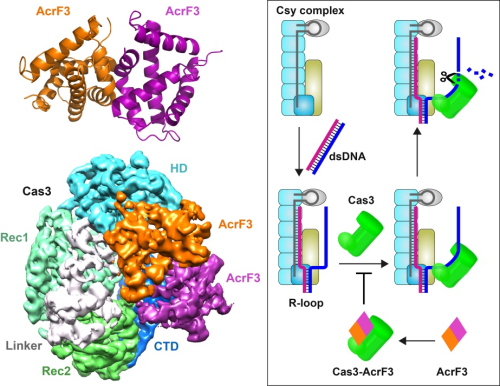
Figure: Crystal structure of AcrF3 and cryo-EM structure of AcrF3-Cas3 (left). The model of AcrF3 inactivating CRISPR/Cas immune system (right) (Image by IBP)

86-10-68597521 (day)
86-10-68597289 (night)

86-10-68511095 (day)
86-10-68512458 (night)

cas_en@cas.cn

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

