
Endocytosis is an energy-using process in which cells absorb macromolecules and particles. The ADP ribosylation factor (Arf) family of small GTPases functions as molecular switches to modulate vesicular and lipid trafficking in cell membrane traffic.
Human BIG1 belongs to the Sec7 domain-containing Arf guanine nucleotide exchange factors (GEFs), which functions mainly on the trans-Golgi to activate Arf proteins from the GDP-bound form to GTP-bound form. Arl1 was suggested to direct BIG1 to the trans-Golgi through interaction with BIG1, and then activate the Arf-dependent pathway in a cascade manner. However, the molecular basis for the specific recognition and recruitment of BIG1 by Arl1 remains unknown.
Prof. DING Jianping’s group from the National Center for Protein Science Shanghai, Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences (SIBS) of Chinese Academy of Sciences, has carried out structural and functional studies of several important proteins involved in the vesicle-mediated transport process.
Recently, Ph.D. candidate WANG Rong and her colleagues in Prof. DING Jianping’s group elucidated the molecular basis for targeting BIG1 to Golgi apparatus by Arl1. This study entitled “Structural basis for targeting BIG1 to Golgi apparatus through interaction of its DCB domain with Arl1” was published in Journal of Molecular Cell Biology.
Researchers determined the crystal structure of the GTP-bound Arl1 in complex with the N-terminal dimerization and cyclophilin binding (DCB) domain of BIG1. In this structure, the DCB domain adopts a HEAT repeat fold, with the four HEAT repeats forming a short arc to embrace Arl1. The Arl1-GTP assumes a typical small GTPase fold and interacts with the DCB domain mainly via the switch I, switch II and interswitch regions. The binding mode between Arl1 and DCB is unique and greatly different from other Arl1-effector complexes.
Throuth structural and biochemical analyses, including GST pull-down and co-localization assays, they revealed several key residues essential for the binding. Also, they analyzed the binding specificity of the BIG1 DCB domain with several Arl small GTPases and identified Arl4a as a binding partner of BIG1.
Based on these results, researchers proposed a working model for targeting BIG1 to the trans-Golgi via interaction of DCB domain with Arl1-GTP.
This study is supported by the National Natural Science Foundation of China and the Chinese Academy of Sciences. The X-ray diffraction data used in the structure determination were acquired at beamline 19U of National Facility for Protein Science in Shanghai.
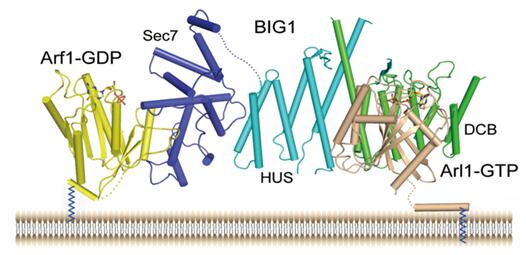
Figure: Working model for targeting BIG1 to the trans-Golgi via interaction of its DCB domain with Arl1-GTP. (Image provided by Prof. DING Jianping's group)

86-10-68597521 (day)
86-10-68597289 (night)

86-10-68511095 (day)
86-10-68512458 (night)

cas_en@cas.cn

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

