
As a typical representative of two-dimensional (2D) materials, graphene has gained extensive attention because the Nobel Prize in Physics in 2010 and led an upsurge study of 2D materials. However, graphene has no bandgap and is limited in the application of semiconductor industry and optical device. Black phosphorus (BP), a new super 2D layered material as graphene, has attracted worldwide attention since its appearance in 2014.
BP is a natural semiconductor with adjustable bandgap and predominant electrical properties, and is considered to be able to replace silicon, and can be the core material of semiconductor industry. The optical property of BP is also superior to other semiconductors. With a direct bandgap, which means the bottom of conduction band and the top of valence band are at the same position, BP could couple with photons directly and construct a new generation of photoelectric device. Besides, BP also has distinctive anisotropic properties in mechanics, such as electrics and thermology.
In spite of these promising properties, what a fundamental obstacle hampering the application of BP is its lack of air-and-water-stability. BP is very reactive to oxygen and water under ambient conditions, which would resulting in compositional and physical changes and consequently considerable degradation in the electronic and optical properties. This caused severe limitation to the research and industrial application of BP.
In order to settle the Achilles' heel of BP, a research team led by professor YU Xuefeng and WANG Huaiyu, from Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, has made great progress recently in preparing BP with high stability over water and oxygen, through a new strategy of surface coordination. BP has a well-known puckered honeycomb structure in which a phosphorus atom is covalently bonded to three neighboring single-layer phosphorus atoms exposing a pair of lone pair electrons. The lone pair electrons can readily react with oxygen to form PxOy, which will transform into phosphate acid in presence of water and lead to the final degradation of BP. Based on this mechanism, the team designed a sulfonic ester of the titanium ligand, which could coordinate with the lone pair electrons of BP through the empty orbit of titanium atom and the strong electrophilic effect of sulfonic ester groups. Due to the occupation of lone pair electrons, BP will not react with oxygen anymore.
The stability study showed that the titanium coordinated BP could keep its optical stability after several days’ exposure in water and in air with a relative humidity as high as 95%, wheras the un-coordinated BP degraded very soon. This modification method is rather simple, which is improving the stability of BP immensely at meanwhile keeping the BP’s crystal structure unchanged. The succusful prepartion of highly stable BP will no doubtly promote the deep research and industrial application of BP in photoelectric device, energy, catalysis and biomedicine.
In the filed of BP reserching, this team has applied one PCT and three Chinese invention patents, and the industrialization of relative works is being processed.
This article titled “Surface Coordination of Black Phosphorus for Robust Air and Water Stability” has just been published as the cover story on Angew. Chem. Int. Ed. The work was supported by the National Natural Science Foundations of China, Leading Talents of Guangdong Province Program, and Shenzhen Peacock Program.
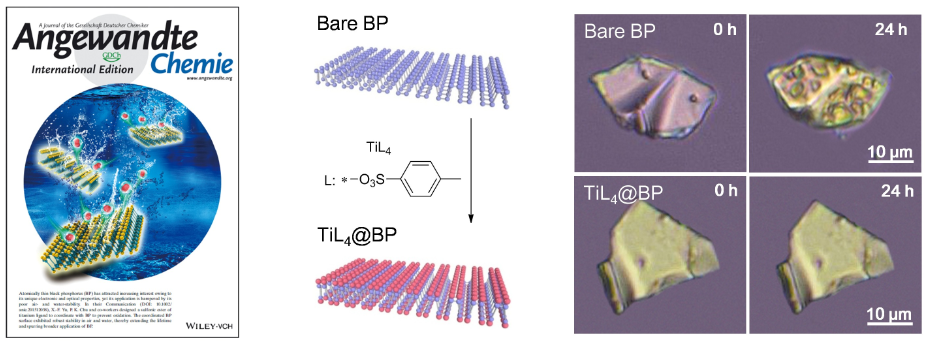
Figure: (Left) cover of Angew. Chem. Int. Ed.; (Middle) preparation of titanium ligand-modified BP; (Right) stability comparison of bare BP and titanium ligand-modified BP (Image By SIAT)

86-10-68597521 (day)
86-10-68597289 (night)

86-10-68511095 (day)
86-10-68512458 (night)

cas_en@cas.cn

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

