
It is marvelous that nature creates isomers which are with same compositions but different structures. These isomers provide excellent materials to understand the structure-property correlation. Isomerism is very common in organic compounds. However, it is rarely reported in nano-particles due to the challenging anatomy of structure at atomic scale.
In 2004, Prof. LU Ke in the Institute of metal research, Chinese Academy of Science (IMR, CAS) found twinning in nano-particles (Science, 2004), which suggests existence of isomerism in nano-particles. In 2007, Nobel Prize winner Prof. KORNBERG and his coworkers reported chirality in Au102 nano-particles by single crystal X-ray crystallography (Science, 2007). TANG Zhiyong’s research group in National Center for Nanoscience and Technology even found chirality in nano crystals (J. Am. Chem. Soc., 2010). In 2008, Prof. GRUENE predicted the existence of isomerism in gas phase clusters (Science, 2008). However, experimental finding of isomerism in metal nanoparticles is unreported so far.
Recently, WU Zhikun and his study team from Institute in Solid State Physics, Chinese Academy of Science (ISSP, CAS) obtained a novel ultrasmall nanoparticle, so-called nanocluster---Au38T, via tuning the reaction parameters, which are exactly the same in composition with that of Au38(SC2H4Ph)24 (abbreviated as Au38Q), reported by Prof. JIN Rongchao’s study team from Carnegie Mellon University in 2010 (J. Am. Chem. Soc., 2010). However, through X-ray crystallography it is found that these two kinds of nanoparticles have different atomic arrangement which thus experimentally confirmed the existence of isomerism in metal nanoparticles for the first time (Figure 1).
Although these two kinds of nano-particles are the same in composition, they show obviously different catalytic activities: Au38T can catalyze reduction of 4-nitrophenol to 4-aminophenol (yield: 44%) at 0℃ in 30 minutes and can be recycled for over 15 cycles as well. Under the same conditions, however, Au38Q could not catalyze the reduction of 4-nitrophenol (Figure 2). Another interesting finding is that the less stable Au38T can be irreversibly transformed to Au38Q at 50℃ (Figure 2). Further investigation are underway in Prof. WU Zhikun’s laboratory.
The collaborators provided data of ESI-MS, Single crystal X-ray crystallography, and theoretical calculation. Graduate student TIAN Shubo is the first author of this paper. Assistant professor LI Manbo conducted the catalytic work. Related findings were published in Nature Communications with title of Structural isomerism in gold nanoparticles revealed by X-ray crystallography. And the study is sponsored by NSFC (21222301), the ministry of science (2013CB934302), Chinese Academy of Sciences (International innovation team) and Hefei Institute of Physical Science, Chinese Academy of Sciences (2014FXCX002).
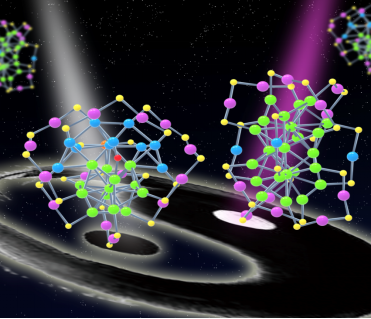
Figure 1. Isomers in nanoparticles: Au38T (left) and Au38Q (right) (Image by LI Manbo)
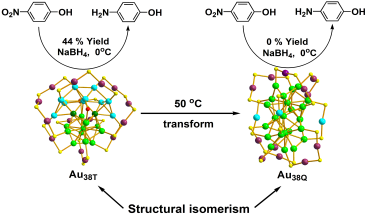
Figure 2. Catalytic activities difference between Au38T and Au38Q, and their inreversible transformation (Image by LI Manbo)
Contact:
Prof. WU Zhikun, Principal Investigator
Key Laboratory of Materials Physics, Anhui Key Laboratory of Nanomaterials and Nanostructures,
Institute of Solid State Physics, Chinese Academy of Sciences
Hefei, Anhui 230000, China
Tel: +86-551-65595395
E-mail: zkwu@issp.ac.cn

86-10-68597521 (day)
86-10-68597289 (night)

86-10-68511095 (day)
86-10-68512458 (night)

cas_en@cas.cn

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

