
G-protein-coupled receptors (GPCRs) are associated with a variety of diseases and hence, have become targets for many modern medications. Glucagon receptor belongs to class B GPCR family and plays crucial roles in the pathogenesis of obesity, glucose intolerance and type 2 diabetes. Two years ago, a joint team led by Drs. Raymond C. Stevens and WANG Mingwei determined the crystal structure of glucagon receptor transmembrane domain that was regarded as a 'landmark’ in GPCR research. However, full-length structures of which as well as other class B GPCRs have yet to be resolved and this hinders our understanding of the relationship between their conformational states and biological functions.
Dr. JIANG Hualiang and WANG Mingwei collaborating with other scientists from Shanghai Institute of Materia Medica (SIMM) of Chinese Academy of Sciences, embarked a study that combines molecular dynamics simulation and functional validation resulting in the discovery of two conformational states for the full-length glucagon receptor. The extracellular domain is stabilized by glucagon binding thereby perpendicular to the membrane surface as ‘open’ state, while in the absence of the peptide ligand, the extracellular surface interacts with the transmembrane domain to adopt a 'closed’ state. The findings, were subsequently confirmed by international efforts.
Based on molecular dynamics simulation of full-length glucagon receptor, scientists proposed a model of dynamic changes involving extracellular and transmembrane domains of the receptor. Using electron microscope, hydrogen deuterium exchange, disulfide bond cross-linking and mass spectrum techniques, the model was validated by receptor binding and functionality assays, thereby revealing the distinct ‘open’ and ‘closed’ conformational states of the receptor. The knowledge gained in this study is of importance for eventual determination of full-length structures, functional analyses and drug discovery of glucagon receptor and other class B G-protein-coupled receptors. This study, published on Nature Communications, demonstrates that glucagon binds to its cognate receptor by a conformational selection mechanism.
This research was accomplished by ten laboratories located in four countries (China, USA, the Netherlands and Denmark) and the Chinese parties received grants from National Health and Family Planning Commission, Ministry of Science and Technology, National Natural Science Foundation, Chinese Academy of Sciences, Shanghai Municipality and Novo Nordisk A/S.
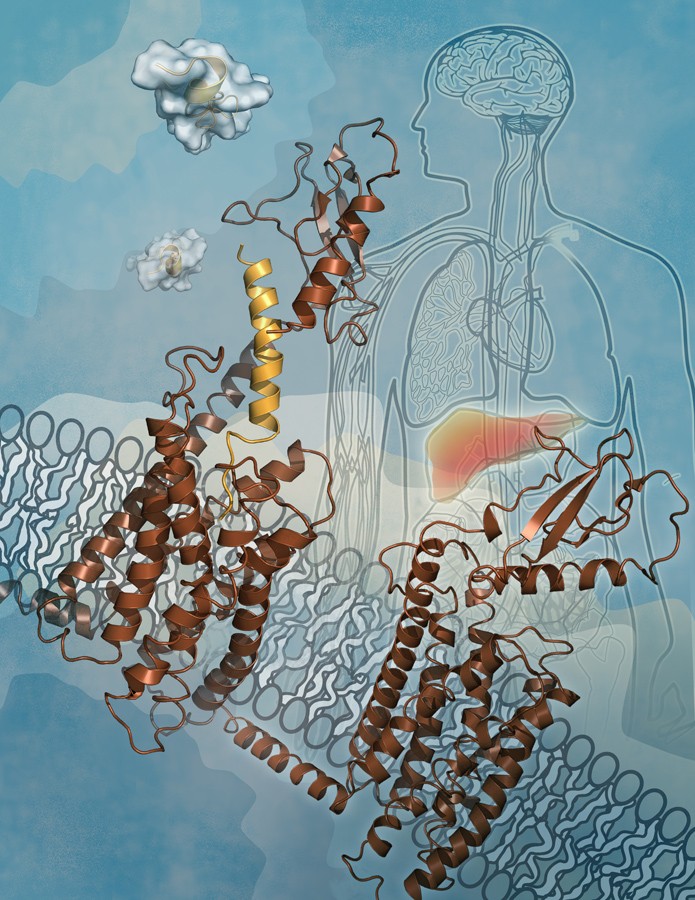
Figure: Conformational change of the human glucagon receptor: open (up left) and close (low right) states. (Image by SIMM)

86-10-68597521 (day)
86-10-68597289 (night)

86-10-68511095 (day)
86-10-68512458 (night)

cas_en@cas.cn

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

