
The hydrogen economy presents a future featuring high energy efficiency and nearly zero pollution. Toyota and Hyundai have started selling affordable hydrogen fuel cell vehicles this year. However, most hydrogen is produced from fossil fuels, such as natural gas and coal, resulting in net greenhouse gas emissions and current hydrogen production facilities equipped with high-temperature and high-pressure reactors require high capital investment and large scale facilities. They cannot be scaled down to produce environmental friendly and affordable hydrogen for local users.
Although a few solutions are proposed to produce carbon-neutral hydrogen in distributed facilities, few solutions are practical considering output, production efficiency, production cost and capital investment. For example, water electrolysis is costly because of high price tags of electricity (i.e., more than 5 cents/KWh) and solar spitting powered by sunlight suffers from slow reaction rate and low photo-to-chemical energy efficiency. Producing hydrogen from less expensive and evenly distributed biomass is an attractive research subject.
Prof. ZHANG Yiheng affiliated with Tianjin Institute of Industrial Biotechnology of Chinese Academy of Sciences, and his co-workers in the USA, have introduced an enzyme cocktail containing more than 15 enzymes which can produce hydrogen from both glucose and xylose of corn stover, one of the most abundant agricultural remains in the world. A commercial cellulase cocktail was used to hydrolyze biomass including cellulose and hemicellulose to glucose and xylose, respectively. The glucose and xylose accounted for more than 95% biomass sugars. This method could produce nearly 12 moles of hydrogen from one glucose and 10 moles of hydrogen from one xylose by using enzyme cocktails, which are three-times of the yield from hydrogen-producing microorganisms.
The researchers from China and USA made breakthroughs in this study. By using the enzyme cocktail instead of microorganism, hydrogen yield was increased two times. By using mathematical modeling and validated predictions, hydrogen production efficiency was optimized with an increase of 67 times to 54 mmole of hydrogen per L per hour. It means that the hydrogen production reaction rate is 15 times of the fastest photo biological hydrogen generation rate and is fast enough for industrial production. Besides, instead of the complicated carbon flux regulation in microorganisms, the enzyme cocktail could utilize both glucose and xylose at the same time. This bioprocess mediated by in vitro artificial enzymatic pathway meets three biomanufacturing criteria (TRY): titer, rate and yield at the same time.
This bioprocess could become the solution to produce affordable green hydrogen from renewable energy resources, which benefits regional social and economic development. The process has advantages like low capital investment and high-purity hydrogen generation without CO. Different from ethanol fermentation which requires high concentration sugar solutions, this process produces and separates hydrogen from low concentration sugar solutions easily which decreases the production cost. Furthermore, the study gave the thinking of biomass serving as a high-density hydrogen storage carrier.
Prof. ZHANG and his co-workers have worked on this project for more than eight years. They believed the benefits of this bioprocess comparing with other hydrogen production methods.
The study entitled “High-yield hydrogen production from biomass by in vitro metabolic engineering: Mixed sugars coutilization and kinetic modelling” has been published in Proceedings of the National Academy of Sciences of the United States of America. A US patent was also issued (US 8,211,861).
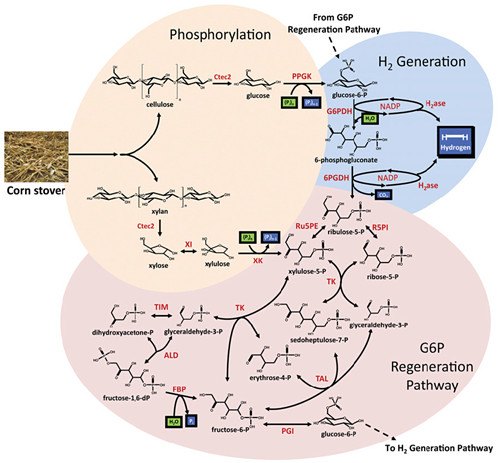
Figure: Pathway depicting the enzymatic conversion of biomass to hydrogen and CO2. (Image by Prof. ZHANG Yiheng‘s group)

86-10-68597521 (day)
86-10-68597289 (night)

86-10-68511095 (day)
86-10-68512458 (night)

cas_en@cas.cn

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

