
T cell signal pathway is almost clear in cytoplasm. But how the antigenic-stimulated signal transmits across the membrane remains largely unknown. Dr. WANG Junfeng’s group from High Magnetic Field Laboratory (HMFL), Hefei Institutes of Physical Science and their collaborators, reported a positive feedback regulation mechanism which amplifies and sustains T-cell receptor activation and should enhance T-cell sensitivity to foreign antigens.
Given the fact that local Ca2+ concentration of the plasma membrane rises quickly in few seconds after initial TCR triggering, this study shows that membrane-proximal Ca2+ can bind to the phosphate group in anionic phospholipids and neutralize their negative charges, which results in the dissociation of CD3ε cytoplasmic domains from the membrane and the solvent exposure of tyrosine residues. As a consequence, CD3 tyrosine phosphorylation is significantly enhanced by Ca2+ influx.
This study demonstrated that calcium functions as secondary messager not only by its generally accepted calmodulin binding property, but also by modulating the electrostatic property of phospholipids which was exemplified for the first time in the TCR activation. This lipid manipulation function of Ca2+ is believed to be applied in understanding other biological processes involving protein-lipd interactions.
Membrane binding proteins are a group of proteins that adhere temporarily to biological membranes. The reversible attachment of these molecules to biological membranes make them ideal participants in cell signaling regulation and many other important cellular events. However, structural studies on membrane proteins lag far behind than those on soluble proteins, and this, in large part, is due to technical difficulties in preparing homogeneous, stable and structurally relevant samples in a membrane-like environment. Phospholipid bilayer Nanodiscs are novel model membranes and have proven to be useful in structural studies on membrane proteins. NMR is a powerful tool to determine the solution structures and characterize the interactions between macromolecules, especially the weak interactions. Combining NMR with Nanodiscs, WANG's lab makes great progress in studying the interactions between membrane binding proteins and membrane. The research results have been published online in Nature (doi:10.1038/nature11699).
This work was collaborated with Dr. XU Chenqi from Institute of Biochemistry and Cell Biology, SIBS, CAS and Dr. LIU Wanli from Tsinghua University and was funded by Chinese Academy of Sciences (Hundred Talents Program), National Basic Research Program of China and National Natural Science Foundation of China.
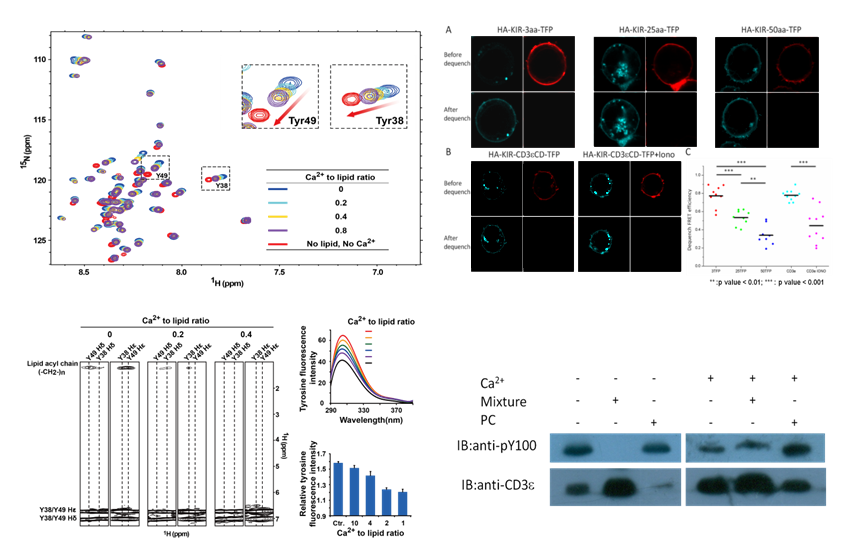
Ca2+ interacts with anionic phospholipids leading to the the disassociation of CD3 cytoplasmic domains from membrane and the consequent CD3 tyrosine phosphorylation (Image by HMFL)
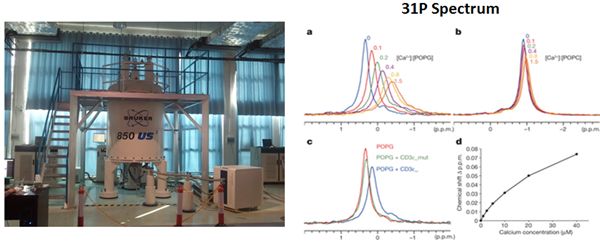
The 850MHz NMR spectrometer at HMFL and the 31P spectra for charactering protein-membrane interactions (Image by HMFL)

86-10-68597521 (day)
86-10-68597289 (night)

86-10-68511095 (day)
86-10-68512458 (night)

cas_en@cas.cn

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

